3. Columns- (Families/Groups)- representative of elements with similar properties-
-analagous
to the number of electrons in outer energy level(s)
a. Representative Elements (A groups) IA- VIIIA- fills s and p orbitals
-IA. Alkali Metals-
-IIA. Alkaline Earth Metals
-IIIA. The Aluminum Family
-IVA. The Carbon Family
-VA. The Pnicogens
-VIA. The Chalcogens
-VIIA. The Halogens
-VIIIA-
Noble
Gases- completely filled
outer s and p orbitals
b.Transition
Metals (B groups)
BI-VIIIB- fills outermost s
(ns) and prior d orbitals
c.
Inner-Transition
Metals- fills outermost s orbital (ns) and twice prior f orbitals
Actinide Series. The elements in the 5f orbitals (botton row of the inner transition metals)
Explanation: -Group Numbering. Explanation of IUPAC and CAS group numbering systems
4. Line of Demarkation: Staggered line used to separate metals from nonmetals
a. metals- elements to the left of the line
b. nonmetals- elements to the right of the line
c. metalloids/weak-metals- elements along the line.
Resource: Metals & Nonmetals
Comparison: Properties of metals & nonmetals
Assignment 1: Section 14.1 Worksheet
Reading Assignment 2: Read section 6.3, questions 18 - 25.
C.
Periodic
Properties of the Elements
Factors that affect the properties:
a. the number of valence electrons
b. the magnitude of the nuclear charge (Z) and the total number of electrons surrounding the nucleus
c. the number of filled shells lying between the nucleus and the valence shell
d. the distances of the electrons in the various shells from each other and from the nucleus
a. Ways to measure the radius of an atom
1.
covalent radius- ˝ distance from nuclei of 2 identical atoms joined by a
single covalent bond
2.
van der Waals radius- ˝ distance from nuclei of 2 atoms of neighboring
molecules
3.
metallic radius- ˝ distance from nuclei of 2 atoms in a solid metal
4
. atomic radius- based on the quantum model. Theoretical/Mathematical approach
b.
Trends in the periodic table
1. Period- radius decreases from left to right- increase in (Z) with same number of energy levels-
2.
Group-
radius increases from top to bottom- increase in the number of energy levels
(Principal Q.N. increases)
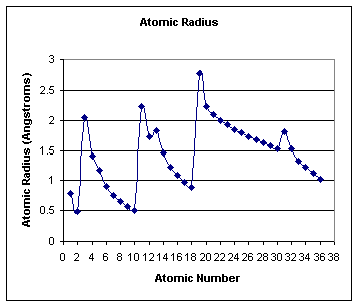
The atomic radii for the elements in the first 3 energy levels
Effective Nuclear Charge (Zeff )- dependent upon (Z) and the shielding effect of other electrons
**
Shielding- interior orbitals that
contain electrons shield the attraction of the nucleus on the valence
electrons.
What effects are seen from shielding?
Slater’s
Rule:
(Zeff = Z –s) where
s
is the shielding factor for valence electrons
calculating
s for
a. for valence electrons in s and p type orbitals
1.
(ns & np) electrons shield at 35%
2.
for (n-1) orbitals, these shield at 85%
3.
for (n-2) orbitals, these shield at 100%
b. for valence electrons in d and f type orbitals
1.
(nd, nf)
shield at 35%
2.
higher orbitals (n+1) shield at 0%
3. s and p electrons in the same energy level (ns & np)and lower energy levels (n-1& et.al.) shield at 100%
a. Ion- an atom which has gained or lost electrons- dependent upon the Effective Nuclear Charge
1. cation- positive ion- due to a loss of electrons
2. anion- negative ion- due to a gain
of electrons
b. Trends
1. Groups- First Ionization energies decrease from top to bottom
2. Periods- General- First Ionization energies increase from left to right
some exceptions occur-
i.
Due to
shielding of ns on 1st electron in a np orbital (B, Al, Ga)
ii.
Losing 1
paired electron is less than losing a parallel electron (O, S, Se)
** these
exceptions fail at higher energy levels** 1st Ionization energy for the elements in the first 3
energy levels
1. First Ionization- removing the first valence
electron
2. Second Ionization- removing the second valence
electron
3. Third Ionization- removing the third valence
electron -removing successive electrons reduces the shielding factor but maintains
Z. This increases the Zeff on the remaining electrons. Resource:
Table of Successive Ionization Energies
3.
Electron
Affinity: The energy change that accompanies the addition of an electron to a
gaseous atom For most atoms, energy is
released when an electron is gained. This is seen as a negative
energy change. General Trend: Atoms that have
a high ionization energy, will typically have a larger negative electron
affinity.
Noble Gases have a positive
electron affinity because it would take energy to add electrons to these
atoms.
Resource: Electron Affinities
--
trends in electron affinity 4.
Ion Size a.
Cations are always smaller than their neutral atom counterpart -losing electrons may:
lose valence shells and/or increase zeff values b.
Anions are always bigger than their neutral atom counterpart. -gaining electrons decrease zeff values c.
Trends-
1.
Period-
Ion size decreases from left to right, (cation/anion specific)
2.
Groups-
Ion size increases from top to bottom. Isoelectric
species- atoms and ions that have the same electron configurations (ex. N-3, O-2, F-1,
Ne, Na+1, Mg +2, Al+3) Octet Rule- All atoms strive to have
full valence shells (typically 8 electrons in the ns & np orbitals)
exception is
1st energy level (no p orbital:
2 electrons) Oxidation Number- the charge of
the stable ion after gaining/losing electrons. Net difference
between protons & electrons 5.
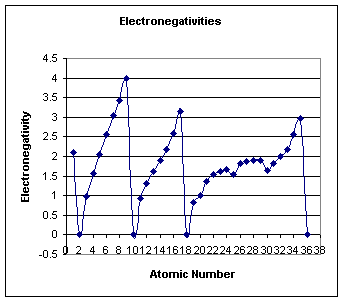
Electronegativity
a.
Trends
1.Periods-
electronegativities increase from left to right
2.
Groups-
decrease from top to bottom.
Applet:
Periodic Table & properties
practice:
Periodic
Table Trends quizzes 1 through 5
Practice
Periodicity
Quiz.
Review
of the Periodic Table: Frostburg State

D. Links