I. Science - The knowledge covering general truth or the operation of general laws especially as commonly obtained and
tested
through the scientific method
A. Scientific Method- A guideline used to pursue answers to many questions or scientific truth.
1. Observation - A description of the physical world
a. Qualitative- a description of the a quality or physical nature
b. Quantitative- a description of quantity or numerical assessment
-these are in the form of counts or measurements
2. Interpretation - An explanation based on observations. Based on personal bias (how we think & what we know).
3. Hypothesis- "Educated Guess"(?)- A tentative explanation based on observations and interpretations that attempts
to
predict future
events. Commonly in
"If/then" format.
-A good hypothesis must be (a) a prediction and (b) testable.
4. Controlled Experiment- A format for testing a hypothesis
a. experimental variable (independent variable)- "What" is being tested
b. experimental group- "Who" is being tested
c. control- variables or groups that are not being directly tested but used as comparison. In a control group, subjects
are exposed to all the same conditions as the experimental group except they are not exposed to the experimental
variable
-the dependent variable is the variable that is being measured as the outcome. It is dependent upon changes in the independent variable
d. data- measurements or observations that are used to arrive at a conclusion based on the hypothesis
-study- an examination of a system in which variables cannot be controlled or manipulated.
A study can be easily misinterpreted, so care must be taken when analyzing the results.
5.
Conclusion- A statement made to either accept or reject the hypothesis
a. Theory- a statement that explains the certain results of a hypothesis
b.
Law- a theory/hypothesis that concisely explains a set of observations
be refuted.
review: To recap the Scientific Method, here is a slide show.
Handout: Here is a printable version of the Scientific Method
practice: Quiz over the Scientific Method.
Reflect: The Nature of Science in the 21st century has expanded to include many research methodologies and technologies. Outline
II. Chemistry
A. What is Chemistry?
-The study of the composition, structure and properties of matter and the reactions by which matter may be formed
or converted into
other
forms.
B. Classification of Matter
Matter- an object that has mass and takes up space (volume)
1. Mixture- Two or more kinds of matter that can be separated by physical
means.
Techniques for physical separations- filtration, straining/sifting, magnetism, & distillation
a. heterogeneous mixture - non-uniform distribution of matter
b.
homogeneous mixture - uniform distribution
solution-
another name for a homogeneous mixture
2.
Pure Substance- matter that cannot be separated by physical processes
a. Element- cannot be broken down into simpler forms of matter
-
chemical symbols- shorthand representation of the elements
Groupings of symbols (a way to help you learn symbols)
i. symbols that don't match elemental names
ii. symbols with single letters that match the
elemental names: (H, B, C, N, O, F, P, S, V, Y, I, U
iii. symbols with 2 letter the match the elemental names: (Li, Be, Cl, Br, Ba, Pt, etc.)
Resource: PubChem- Periodic Table of Elements with properties
Practice: Learning your symbols.
b. Compound- pure substances that can be broken down into simpler forms only
by chemical processes.
-
chemical formula- a group of chemical symbols representing a compound
Sodium chloride (salt)- NaCl (1
sodium and 1 chlorine)
Carbon dioxide - CO2 (1
carbon and 2 oxygen)
handout.
Classification of Matter
For Additional Information pertaining to States of Matter
C.
Properties of Matter
1. Physical Properties- characteristics of matter that can be measured or observed without changing the
composition of the matter.
a.
intensive properties- mass independent properties
-color,
shape, composition, shape, taste, smell, etc.
i.Temperature-
the average heat energy (kinetic) in a substance
-Fahrenheit
Scale- (32o - 212o) degrees
-Celsius Scale-
(0o-100o) degrees
** Fahrenheit and Celsius are standardized by the melting and boiling points of pure water at a certain
atmospheric pressure.
-Kelvin
Scale-
** absolute zero- point where no heat energy exists within in an object**
practice. Temperature conversions
| TF= 1.8*TC + 32 | TC = (TF-32) / 1.8 |
|
TK = TC + 273.15 |
TC = TK - 273.15 |
|
Is there a temperature where the Celsius and Fahrenheit temperatures are numerically the same? Click here. |
|
ii.
Density- mass per unit volume-
"apparent heaviness"
**density of water = 1.0 g/cm3 @ 4 oC.
|
Density = Mass Volume |
Practice. Calculating Densities
iii.
Specific gravity- ratio of density of matter to density of water
** specific gravity has no units.
Chart listing the specific gravity of common materials
|
Specific Gravity = Density of Material Density of Water |
b. extensive properties- mass dependent properties
i.
heat (Q)- the total amount of heat energy (kinetic energy)
-calorie- amount of heat that
increases 1 gram of water by 1 oC.
-joule- 1 calories = 4.184
practice: extensive and intensive properties.
c. States of Matter-
|
State |
shape |
volume |
internal heat |
|
1. Solid |
uniform |
uniform |
lowest |
|
2. Liquid |
variable |
uniform |
medium |
|
3. Gas |
variable |
variable |
high |
2. Chemical Properties- characteristics that describe the ability of matter to undergo changes in composition
where new substances are formed with new properties.
ex.
flammable/combustible, reacts with acids/bases, decomposes under light/uv
(light labile), etc.
D. Changes in Properties- (Video)- Physical vs chemical changes
1.
Physical
Changes- A change in physical properties of matter without a change in the
chemical composition
a.
Melting/Freezing - Solid
/ Liquid
b. Evaporation/Condensation
- Liquid /
Gas conversion
c. Sublimation - Solid / Gas conversion
2. Chemical Changes- A change in matter that produces new substances with
new properties (chemical reaction)
a.
reactants- substances that undergo changes
b.
products- new substances that are formed
precipitate- a solid produced from solution during
a reaction
c.
Ten signs of chemical change
1. Bubbles of gas appear
2. A precipitate forms
3.
A change in color
4.Temperature changes
5.Light is emitted
6.A change in volume
7.A change in electrical conductivity
8.A change in melting or boiling point
9.A change in smell or taste (not to be performed in lab)
10.A change in chemical or physical properties
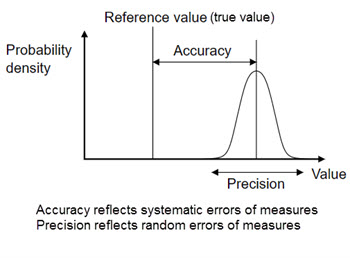
1.Accuracy vs. Precision
Accuracy-
the closeness of a measurement to the actual value
Precision-
the reproducibility of a measurement

2. Significance of Measurement
-all
measurements contain exact digits and 1 estimated digit
a. Significant Figure Rules
1. Non-zero digits are always significant.
2. All zeros between other significant digits are significant.
3. The number of significant figures is determined starting with the leftmost non-zero digit. The leftmost non-zero digit is sometimes
called the most significant digit or the most significant figure. For example, in the number 0.004205 the '4' is the most
significant figure. The righthand '0's are not significant. The zero between the '2' and the '5' is significant.
4. The rightmost digit of a decimal number is the least significant digit or least significant figure. Another way to look at the
least significant figure is to consider it to be the rightmost digit when the number is written in scientific notation. Least significant
figures are still significant! In the number 0.004205 (which may be written as 4.205 x 10-3), the '5' is the least significant figure.
In the number 43.120 (which may be written as 4.3210 x 101), the '0' is the least significant figure.
5. If no decimal point is present, the rightmost non-zero digit is the least significant figure. In the number 5800, the least significant figure is '8'.
b. Atlantic/Pacific Rule for Determining Significance
** If decimal is Present (Pacific), come in from the left and stop at the first nonzero digit. The remaining places are significant.
** If decimal is Absent (Atlantic), come in from the right and stop at the first
nonzero digit. The remaining places are significant
Note: If there are zeros in a number that are significant but can't be represented separately from non-significant zeros, then we
example: Represent the measure 2,300 ft that is significant to the tens but not the units.
= 2.30 x 103 ft (Answer has only 3 significant figures. Instrument was accurate to the hundreds of feet)
Image: Which figures are significant
Practice. Identifying Significant Figures
Image: Significant Figures in measures
3.
Significant figures in calculations
a.
addition/subtraction- The answer will contain the last significant place as the
number with the least accurate significant place.
example:
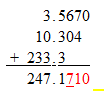 |
The tenths is the last significant place in 233.2, which means the answer cannot be more accurate than the least accurate place.
Answer would then be 247.2 The 7 rounded the 1 to a 2 in the tenths place
|
b.
multiplication/division- an answer will contain the same number of sig figs as
that least number of sig figs in any number
example
 |
Answer is 480 or 4.8 x 102. (answer can only have 2 sig figs) The 8 rounded the 7 up to an 8.
|
4. Uncertainties in measurements.
-All measurements have inherent uncertainties, whether due to limitations in accuracy, calibration errors or human errors. These measurement errors are defined as:
a. Random errors- These are due to accuracy limitations of the measurement instrument. The error will either create a larger or a smaller measure
than the actual measure, dictated merely by chance. Random errors are reduced through replication (taking multiple measures) and calculating mean values.
Examples of random errors:
Uncertainity of the instrument
The effects of environments factors such as temperature or air currents which can alter measures
insufficient data
observer misinterpretation of the data/measurement
--The uncertainty of random errors can either be calculated or it can be defined as the range of measures 1/2 the smallest measurement division (level of accuracy)
| Example of uncertainty of measures | ||
|
|
The level of accuracy of the graduated cylinder is
to the nearest 1 mL. We can't be certain of any measurement smaller
than to the nearest 1 mL. According to our sig fig rules, we can
"guess" one place past the smallest division of measure. The meniscus is sitting past the third division past 40 mL, so we would say it is approximately 43 mL, without be more certain than that. Therefore, we can estimate how much past it is. This becomes our last sig fig. The measure could be given as 43.5 mL with the understanding the last sig fig is always our estimate. But how about the uncertainty? The uncertainty of this measure would be defined as 1/2 the smallest division, which would be 1/2 of 1 mL = 0.5 mL. So, the true measure should lie either 0.5 mL above or 0.5 mL below our measurement. We would say then our measure is 43.5 + 0.5 mL. Remember that all measurements contain three parts:
|
|
The level of uncertainity is dependent upon the instrument:
1. Analog instruments (have a scaled readout) have uncertainities of +1/2 the smallest division
2. Electronic instruments (digital readout) have uncertainities equal to + the smallest division
3. Calibrated instruments (from the manufacturer) may have pre-calibrated levels of uncertainty.
b. Systematic errors- These are errors that cause the measurement to be either always larger or always smaller than the actual measurement. This affects
affects the accuracy of a measure.
| Examples of Systemic errors: | |
|
Practice: Taking measurements
c. Basic rules for propagation of uncertainties
|
|||||||||||||||||||||||||||||||||||||||||
Video: Review of Measurements, uncertainties & propagation (IB Course)
5. Metric System- (SI
system-
International System of Units-c.1960)
Standards for measurements used by scientists which have become more precise as technology has changed. New revisions, 2019
|
Physical Quantity |
Name of Unit |
Abbreviation |
|
Mass |
Kilogram |
kg |
|
Length |
m |
|
|
Time |
Second |
sec (or s) |
|
Electric Current |
Ampere |
A |
|
Temperature |
Kelvin |
K |
|
Luminosity |
Candela |
cd |
|
Amount of Substance |
Mole |
mol |
-The mole is a standard unit for an amount of any material. It is equivalent to 6.022 x 1023 particles. This is commonly called Avogadro's Number (N or N0)
- Molar mass is the mass of 1 mole of any material. Commonly measured in grams/mole (g/mol)
- Molar volume is the volume of 1 mole of any gas at Standard Temperature & Pressure (0o C and 1 atm)
Video: What is a mole? TedEd
Image: Mole map
a. All
metric measurements are related by factors of 10
-this avoids confusion when converting from one unit measure to another.
|
Prefixes used in the SI System |
|||
|
Prefix |
Abbreviation |
Power of 10 |
Value |
|
Tera- |
T |
1 x 1012 |
1000000000000 |
|
Giga- |
G |
1 x 109 |
1000000000 |
|
Mega- |
M |
1 x 106 |
1000000 |
|
Kilo- |
k |
1 x 103 |
1000 |
|
(Base) |
(gram, meter, liter,etc.) |
1 x 100 |
1 |
|
Deci- |
d |
1 x 10-1 |
0.1 |
|
Centi- |
c |
1 x 10-2 |
0.01 |
|
Milli- |
m |
1 x 10-3 |
0.001 |
|
Micro- |
m |
1 x 10-6 |
0.000001 |
|
Nano- |
n |
1 x 10-9 |
0.000000001 |
|
Pico- |
p |
1 x 10-12 |
0.000000000001 |
|
Femto- |
f |
1 x 10-15 |
0.000000000000001 |
examples:
1 Megameter = 1 x 106 meters or 1,000,000 meters
1 milliliter = 1 x 10-3 liters or 0.001 liters (also thought of as 1 liter = 1000 mL)
** To define the conversion between two units, calculate the powers of ten difference, thus the conversion.
ex. The difference between nano- and milli- is 6 powers of ten.,
therefore the conversion is 1 milligram = 1,000,000 nanograms
6. Problem Solving with measurements.
a. Equality- a statement of two things being equal
ex. 1 foot = 12 inches
b. Conversion- an equality written as a fraction.
- a conversion has the value of 1. What happens when you multiply something by 1?
1 foot
12 inches
c. Unit Analysis (Dimensional Analysis)
- a format for solving problems. The advantage is that it organizes your math problems and allows you to cancel out units as
you progress through the problem.
Practice & Tutorial: Conversions practice. There are problems at the bottom and answers for each.