Brønsted-Lowry Acid- Substance that increases [H+]
Brønsted-Lowry Base- Substance that decreases [H+]
Lewis Acid- Electron pair acceptor (electrophile)
Lewis Base- Electron pair donor (nucleophile)
pH = -log[H+] ; higher [H+] = lower pH
Reactions can be readily reversible. A reversible reaction where the forward and reverse
reactions occur at the same rate is said to be at equilibrium
A + B D C + D
Equilibrium Constant Keq = [products]
[reactants]
|
for self-ionization of water: H2O D H+ + OH-
Keq = [ H+ ] [OH-] for [H2O] = 1000g/L = 55.5 M [H2O] 18 g/mol
given that [ H+ ] = [OH-] = 1.0 x 10-7 M
then Keq = (1.0 x 10-7)( 1.0 x 10-7) = 1.8 x 10-16 55.5 therefore [ H+ ] [OH-] =(Keq )(55.5) = (1.8 x 10-16)(55.5) = 1.0 x 10-14 = Kw
|
Kw à Ion Product of water. This describes that the concentration of hydrogen ions
and hydroxide ion are inversely proportional.
Can we calculate pH when [ H+ ] = [OH-]
pH = - log [ H+ ] = - log (1.0 x 10-7) = 7
So, when [ H+ ] = [OH-] , the solution is neutral.
Ionization Constant (Ka)- describes the ability of an acid to form ions in solution.
For strong electrolytes: ( like strong acids)
HCl + H20 à H3O+ + Cl-
[ HCl ][ H20 ]
So… this says that in solution, the prevalent forms are H3O+ and Cl-
Weak Electrolytes. Do they strongly dissociate?
Resource: Strength of acids & bases
Resource: Ka values for weak acids & Kb values bases
Acetic Acid (CH3COOH)
CH3COOH + H2O à CH3COO- + H3O+
Keq = [CH3COO- ][H3O+] , but with weak electrolytes, [H2O] remains
[CH3COOH][H2O] very large and almost unchanged. IGNORE IT.
Ka = [CH3COO- ][H3O+] = 1.74 x 10-5
[CH3COOH]
So what does this mean?
Relating pH and Ka values. The Henderson-Hasselbalch Equation
Ka describes the equilibrium concentrations of the ionic forms and acid forms.
What does pH depend on?
The amount of H+ in solution is dependent upon the amount of acid that has been
dissociated.
In other words [H+] = [A-], given the equation: HA + H2O à H3O+ + A-
Deriving Henderson-Hasselbalch,
Ka = [A- ][H3O+]
[HA]
[H3O+] = Ka [HA]
[A- ]
Take (-log) of both sides pH = pKa - log [HA]
[A-]
pH = pKa + log [A-]
[HA]
Can we find the pH when a weak acid is ½ dissociated if we know the Ka ?
pH = -log Ka when [A-]= [HA], because if [A-] = [HA], then log 1 = 0
pH of HAc (@ [Ac-] = [HAc]) = - log (1.74 x 10-5) = 4.76
Does the initial concentration of HCl affect the pH?
Does the initial concentration of HAc affect the pH?
Changes in pH as a function in changes in [A-] or [HA]
pH = pKa + log [A-] à pH = pKa +(-2)
[HA]
therefore: log [A-] = -2
[HA]
(take 10^ of both sides) [A-] = 0.01
[HA]
What does this mean?
The pKa of Formic Acid is 3.75. Calculate using the Henderson-Hasselbalch equation the pH of a solution containing 200 mL of 0.1 M Formic acid and 150 mL of 0.1 NaOH with enough water bring the entire solution volume to 1 L.
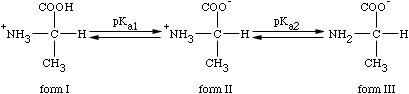
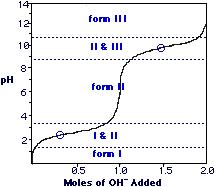
Titrations of Amino Acids
 |
|
|
Solving for Isoelectric point
pI = pKa1 + pKa2
2
For alanine, the pI equals
pI = 2.3 + 9.7 = 6 2
So, what does this mean?
|
 |
|
|
|
|
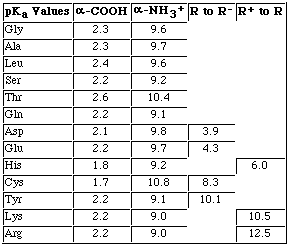
Calculate the pI for Glutamic Acid.