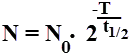Unit II. Nuclear Chemistry
Reading Assignment 1:
Read & KTU Ch. 25.1-2. Answer questions 1-16
I.
Chemical
reactions vs. nuclear reactions.
A. Chemical Reactions
-
Matter undergoes a change in composition where atoms are rearranged by
breaking and making chemical bond
-
These reactions are affected
by temperature, pressure, catalysts and concentrations of reactants.
-
The type and number of atoms are
conserved in a normal chemical reaction (Law of Conservation of Matter):
-
If the matter is conserved, then mass is conserved
which means -- Mass of Reactants = Mass of Products
B. Nuclear reactions.
II.
Radioactivity
A. Nucleons- The
components of the nucleus. Protons (11p+)
and Neutrons (10n0)
1. atomic number (Z)- the number of protons
2. mass number (A)- the number of nucleons in an atom (protons + neutrons)
3.
isotopes- atoms of the same elements that differ by the number of
neutrons
a. nuclide- the nucleus of specific isotope of a certain element
4. radioisotope- an isotope that contains an unstable nuclide.
a. radionuclide- the unstable nucleus of a radioisotope
Video:
Radioactivity-Expect the Unexpected
Resource:
The
story of how radioactivity was discovered --
timeline
Resource:
The
theory behind radioactivity and nuclear stability
B. Energy & mass-- Mass defect
Question: What comprises the helium atom?
-
2 protons (@ 1.0073 a.m.u), 2 neutrons (@1.0087 a.m.u.) and 2 electrons (@
0.00055 a.m.u)
-
The pieces of the helium atom add up to 4.0331 a.m.u.
--Isotopes
of Helium
-
But according to the periodic table, helium has a mass of 4.0026.
Where does the other 0.0305 a.m.u. go?
1. Mass Defect- the difference in masses between the atom and the sum of the
atom's components.
2. Nuclear Binding Energy- the energy associated with the mass defect
defined by Einstein's equation (E = mc2)
|
Example:
Calculate the binding energy for the Helium atom (42He) |

Total mass doesn't include electron masses but the total mass defect
is the same. |
|
|
1.
Calculate the mass defect |
|
4.0026
a.m.u. - 4.0331 a.m.u. = -0.0305 a.m.u.
|
|
2.
Calculate the nuclear binding energy from E = mc2 ( 1 a.m.u.
= 1.6605 x 10-27 kg) |
|
E
= (-0.0305 a.m.u.* 1.6605 x 10-27 kg/ 1 a.m.u.) * (3.00 x 108
m/s)2
= -4.55 x 10-12 kg m2/s2
What
is a kg m2/s2? It is the same thing as
a joule (unit for energy)
-4.55
x 10-12 J. This doesn't seem like much energy
(basically 4 trillioniths of a joule).
But remember this is for one atom. But what about many atoms?
4.0026
grams of Helium contains approximately 6.022 x 1023 atoms
(This is called Avogadros number: see mole)
So,
-4.55 x 10-12 J/atom * 6.022 x 1023 atoms
= -2.74 x 1012 J (This is over 2 trillion
joules for just 4 grams)
ex. 1000 ton meteorite releases 5 x 1013
J. (1 kiloton TNT = 4.2 x 1012 J, Hiroshima atomic bomb=6.3 x
1013 J) |
C. Types of Radioactive particles
All radioactive decay reactions
can be thought of as an unstable parent nuclide decaying into a daughter
nuclide.
1. Alpha Particles. High speed
helium nuclei. written as 42He or 42a.
Associated with heavy isotope
decay (N > 83 and A >= 200)
ex. 21284Po
----> 20882Pb + 42a
Polonium was the first
radioactive element found. Discovered by Marie Curie and her husband
Pierre in 1898.
20882Pb is
the most abundant isotope of lead (~52.4%)
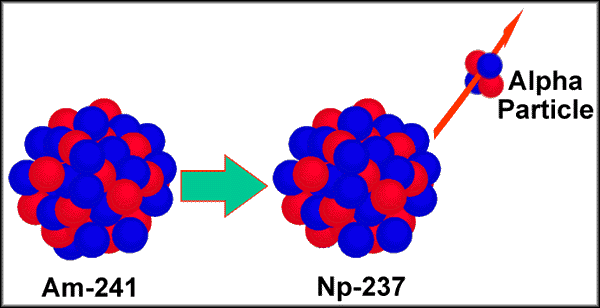
The alpha decay of 241Am
(americium-241) to form 237Np (neptunium-237)
2. Beta Particles. High speed
leptons (electrons); written as 0-1e- or b
Associated with neutron
decay. 10n ---> 11p
+ 0-1e-
ex. 9843Tc
---> 9844Ru + 0-1b-
Technetium is a radioactive
element that does not occur naturally but instead was artificially prepared
in 1937
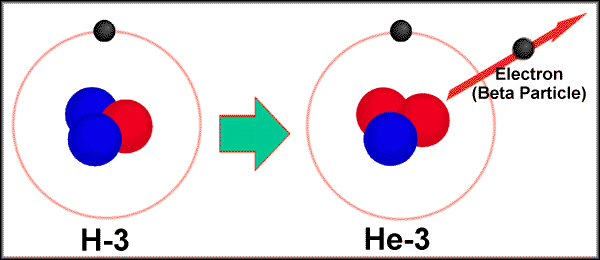
Tritium (31H) decaying into 32He
Question: What is Tritium?
Click
here to read about it.
Resource:
Rutherford's
discovery of the alpha and beta particles
Practice:
Exercises
in writing alpha and beta decay equations.
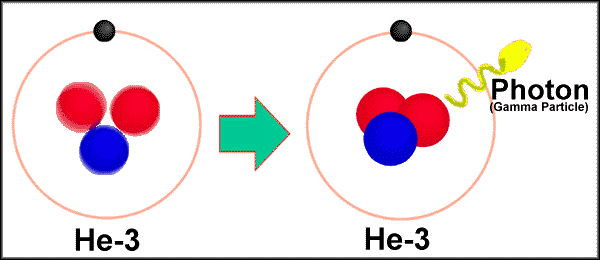
3. Gamma radiation.
Electromagnetic radiation with high frequency and high energy. written as g
Usually accompanies all
radioactive emissions. Represent lost energy.
An interesting gamma
emission. The annihilation of an electron and a positron forms gamma
radiation:
0-1e-
+ 0+1e ----> 2g
( 0.511 MeV or 8.187 x 10-14 J) ( 1 Megaelectron volt
= 1.602189 x 10-13 J)
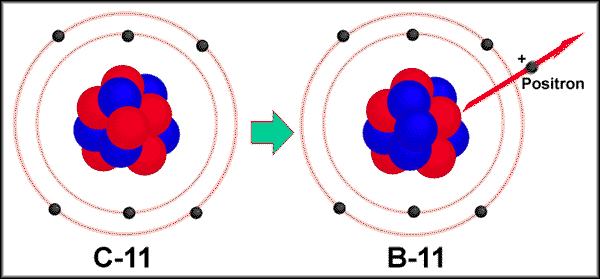
4.
Positron
emission- Particle that is similar to an electron but with a
positive charge. written as 0+1e or b+
Positron emission essentially
converts a proton into a neutron. 11p
----> 10n + 0+1e
ex. Carbon-11
decay. 116C
---> 115B + 0+1e.
Resource:
Positron
annihilation studies at the University of Bristol, UK.
5. Electron capture- The nucleus
captures an n=1 electron.
Electron capture produces an
effect similar to positron emission, converting a proton to a neutron.11p
+ 0-1e- ----> 10n
ex. 74Be
+ 0-1e- ---> 73Li.

Practice:
Exercises
in writing positron emission and electron capture equations
Applet:
Identifying
Radioactive Decay Series
D. Patterns of Stability
1. Neutron-to-Proton ratios
Nucleons are held together by
Strong Nuclear Forces.
The stability of the nucleus is
dependent upon the neutron-to-proton ratio. As Z increases the number of
neutrons needed also increases but not in a linear relationship.
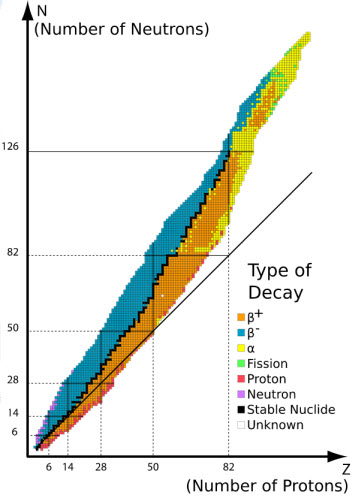
a. Belt of Stability. A
region on a neutron to proton graph that represents stable nuclides
Notes to Consider:
1. All nuclides with Z>83 are
unstable
2. Z values lower than 20 have
neutron/proton = 1
3. As Z increases above 20, the
neutron/proton ratio increases in stable isotopes. ex. 90Zr = 1.25, 120Sn
= 1.4, 200Hg = 1.5
4. Region above the belt
represents excess neutrons
5. Region below the belt
represents excess protons
Predicting decay based on neutron-to-proton ratio
Notes to consider:
1. Above the Belt: Represents high
neutron/proton. Seen as Beta Emission. Increases Z and A remains same
2. Right of the Belt Represents
Z>83. Alpha particles emitted to reduce both A and Z
3. Below the Belt: Represents
low neutron/proton. Positron emission or electron capture. Decreases Z
and A remains same
Assignment 1:
Radioactive decay equations
worksheet
E. Rates of Radioactive Decay
1. Half-Life: The time to
decay 1/2 of a sample of radioactive nuclides into their stable daughter
nuclides
a. Decay rates are measured in
disintegrations per time. Also known as a sample's activity
Video:
Half life (Bill Nye)
| Decay rates &
determining ages |
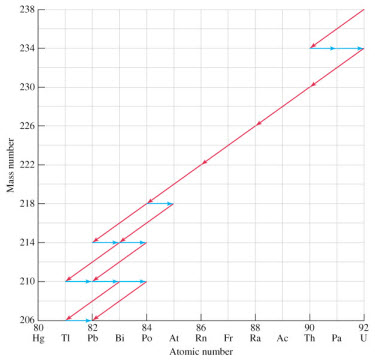
| R =
k N |
R is activity (disintegrations per time), k
is a decay constant, and N is the number of nuclides. |
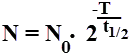 |
N = number of undecayed nuclei after time
(T)
N0 = number of original radioactive nuclei
T =
time which decay has occurred
t1/2 = half-life of
specific isotope |
Applet:
Identifying
the rate of decay.
Practice:
Looking at decay rates
Resource:
List of radioactive isotopes & half-lives --
by half-lives

Resource:
Law of
Radioactive Decay. Relates the amount of decayed radionuclide to
undecayed radionuclide
b.
Radioactive
Dating
A comparison of radioactive
nuclei to the stable daughter nuclei in an artifact can predict the age.
Comparing the ratio of
radioactive to stable nuclei in the same and then in the environment,
scientists can infer the age by determining the number of half-life
disintegrations.
Resource:
What
is carbon-14 and how is it produced?
Resource: What is
Carbon-dating?
Resource:
Radioactive
Decay Calculator
Table:
Naturally
occurring isotopes and half-lives.
Assignment 2 : Small-Scale lab, pg 887
b. Transmutation reactions.
Creating unstable nuclei from
stable nuclei through bombardment of neutrons or other nuclei
The first transmutation was
performed by Ernest Rutherford in 1919. Bombarding 14N with alpha
particles
147N
+ 42He ---> 178O
+ 11H
** Both the oxygen-17 and
hydrogen-1 are stable so there is no further decay.
c. Nuclear decay series. Nuclides with Z much
larger than 83 cannot decay to stable nuclides with one emission. This
usually requires multiples steps representing multiple decay emissions.
Applet:
An applet showing
decay series of some transuranium
elements
Resource:
What are the
transuranium elements?
Resource: A historic
film showing transuranium elements. Hosted by Glenn Seaborg
reading asssignment 2: Read & KTU Ch.
25.3-25.4, answer questions 18-33
III. Nuclear
Fission and Fusion
1.
Nuclear Fission.
The splitting of heavy nuclei that results in the release of energy.
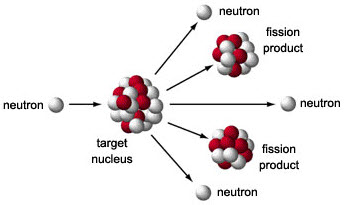
-Critical
mass can lead to a
nuclear chain reaction
Video:
Modern
Marvels--The Manhattan Project
2.
Nuclear Fusion. The union of
nuclides forming larger nuclide and the release of energy
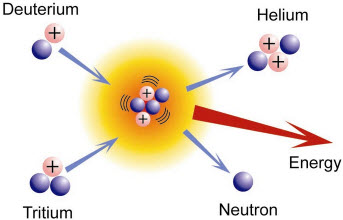
Resource:
Video
tutorial of Fission and Fusion and the applications.
Resource:
Nuclear
Fusion in the Sun
Assignment 3: Standardized Test Prep pg. 905,
1-17
Resource:
Common uses for radioisotopes --
The regulation & use of radioisotopes
References: The types of decay diagrams are adapted from Thinkquest