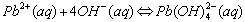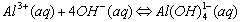A complex ion is an ion that contains a metal cation bound to one or more small molecules or ions (such as NH3, CN- or OH-). These are usually formed from a transition metal surrounded by ligands (polar molecules or negative ions). As a "rule of thumb" there are twice the number of ligands around a cation as the charge on the cation.
Example: the dark blue Cu(NH3)42+ (ammonia is used as a test for Cu2+ ions), and Ag(NH3)2+.
Memorize the common ligands.
| Ligands | Names used in the ion |
| H2O | aqua |
| NH3 | ammine |
| OH- | hydoxy |
| Cl- | chloro |
| Br- | bromo |
| CN- | cyano |
| SCN- | thiocyanato (bonded through sulphur)
isothiocyanato (bonded through nitrogen) |
Watch out for:
-
Aluminum also forms complex ions as do some post transitions metals. Ex: Al(H2O)63+
-
The names are similar to writing ionic compounds. Cu(NH3)42+ is the tetraamminecopper(II) ion, Ag(NH3)2+ is the diamminesilver(I) ion, and Al(H2O)63+ is hexaaquoaluminum(III) ion. Zn(OH)42- is the tetrahydroxyzinc(II) ion, the charge is the sum of the parts (2+)+4(-1)= -2.
-
Acid-base reactions may change NH3 into NH4+ (or vice versa) which will alter its ability to act as a ligand.
-
Visually, a precipitate may go back into solution as a complex ion is formed. For example, Cu2+ + a little NH4OH will form the light blue precipitate, Cu(OH)2. With excess ammonia, the complex, Cu(NH3)42+, forms.
-
Keywords such as "excess" and "concentrated" of any solution may indicate complex ions. AgNO3 + HCl forms the white precipitate, AgCl. With excess, concentrated HCl, the complex ion, AgCl2-, forms and the solution clears.
-
The odd complex ion, FeSCN2+, is one that is commonly used in standard complex ion problems.
-
Transitional metals, such as Iron, Zinc and Chromium, can form complex ions. Aluminum can form complex ions as well.
Cations that form complex ions with excess NH3 are:
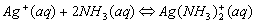
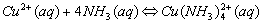
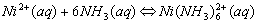
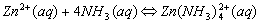
Cations that form complex ions with excess OH- are: