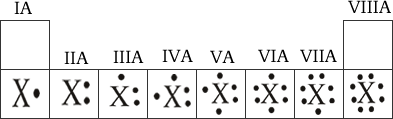
Valence electrons determine the chemical reactivity of the elements. As we saw in the last unit, elements of the same family share the same number of valence electrons and therefore the properties of the elements are dependent upon these electrons. Electron-dot structures are tools that we used to show the number of valence electrons for each element.
1. Valence electrons are drawn as dots around the elemental symbol
2. The s & p orbitals contain the valence electrons and therefore the electron-dot structures conserve this model
3. Here is what a general electron-dot structure looks like.

- remember that electrons in the same orbital or subshell possess the same energy (i.e. degenerate) so their position on the electron dot structure can interchange with another electron of the same energy.
The following shows general electron-dot structures for elements within the main groups.