| Sodium atom | Sodium ion |
| electron configuration: 1s2 2s2 2p6 3s1 | electron configuration: 1s2 2s2 2p6 |
| electron-dot structure:
|
electron-dot structure:
|
| symbol with oxidation state: Nao | symbol with oxidation state: Na+1 |
Oxidation numbers indicate the general charge of an atom after it has either gained, lost, or shared electrons to be stable. Even though atoms that share electrons don't "lose" electrons, they do have the ability to share different numbers of electrons.
1. Metals will typically lose electrons to achieve stable configurations, due to their lower Zeff values. The result are positive ions (fewer electrons than protons) which are called cations.
| Sodium atom | Sodium ion |
| electron configuration: 1s2 2s2 2p6 3s1 | electron configuration: 1s2 2s2 2p6 |
| electron-dot structure:
|
electron-dot structure:
|
| symbol with oxidation state: Nao | symbol with oxidation state: Na+1 |
** Notice the electrons in RED, are the same as the electrons in the electron-dot structures.**
2. Nonmetals will typically gain electrons to achieve stable configurations, due to their high Zeff values. The result are negative ions (more electrons than protons) which are called anions.
| Sodium atom | Sodium ion |
| electron configuration: 1s2 2s2 2p6 3s2 3p5 | electron configuration: 1s2 2s2 2p6 3s2 3p6 |
electron-dot structure:
 |
electron-dot structure:
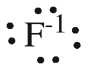 |
| symbol with oxidation state: Fo | symbol with oxidation state: F-1 |
** Notice the electrons in RED, are the same as the electrons in the electron-dot structures.**