Unit VII Notes. Structure & Properties of Water
1. Structure of Water

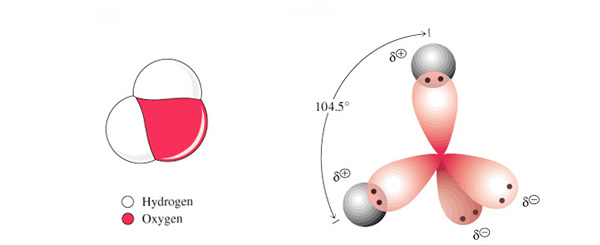
Water has a VSEPR of AX2LP2. The tetrahedral arrangement of peripheral atoms (bond angles of 109.5o) shows contraction of the bond angle due to the lone pair electrons proximity to the central atom (oxygen). The difference in electronegativity (3.5-2.1 = 1.4) identifies each H-O bond as a Polar Covalent bond. The charge asymmetry found in water (electron withdrawing from each hydrogen) creates a polar molecule.
2. Hydrogen Bonding of water

The electrostatic attraction between the partially positive hydrogen atoms and adjacent partially negative oxygen atoms allows water molecules to interact. This Hydrogen Bonding is considered one of the 4 intermolecular bonds that can occur between particles. These interactions cause water to have a higher "apparent" mass. This increase is seen as an increase in boiling point and decrease in vapor pressure.
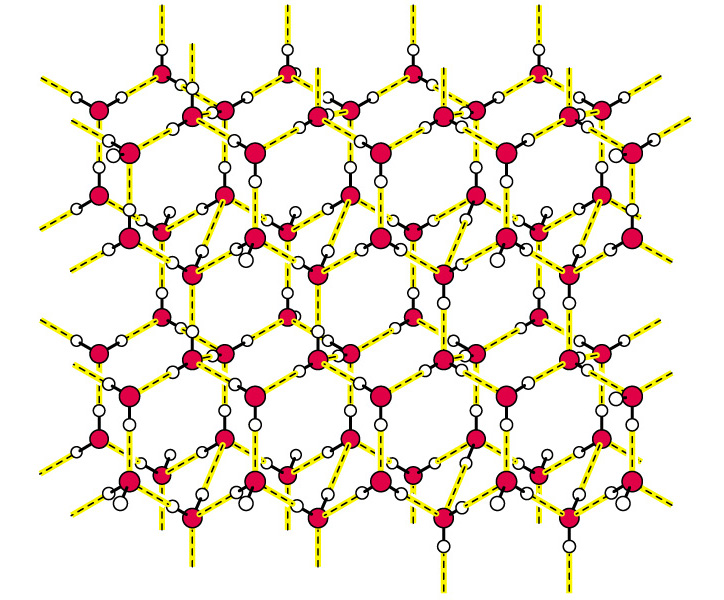
3. Arrangement of water molecules due to Hydrogen Bonding.
| H-Bonding of an individual water molecule | H-Bonding of water in ice. |
|
|
 |