
Unit VII. Thermodynamics & Chemical Reactions Ch. 5 & 19
I. Chemical Reactions & Thermodynamics: A reaction is a process where matter undergoes a change in composition. This change
in composition is commonly associated with a change in the energy states of the materials.
-Thermodynamics is the study of energy and the ways it can be transformed in a system
-Thermochemistry deals with how energy specifically changes within a system undergoing a chemical change.
A. Energy- A characteristic of an object as defined it's ability to do work or transfer heat.
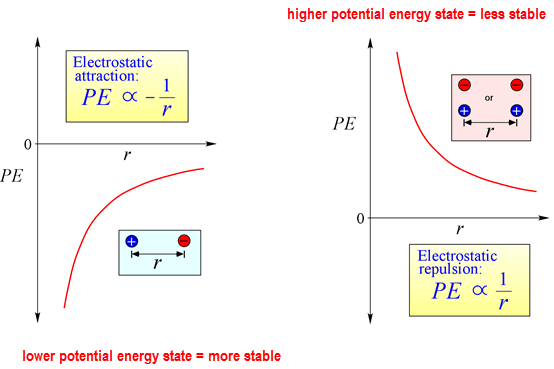
1. Potential Energy is the energy associated with the position or location of an object relative to another.
Ex. book on a desk or electrons in energy levels.

Point charges can create a force of attraction or force of repulsion
The work done to move particles closer can either increase energy (like charges) or reduce energy (opposite charges)
2. Work is defined by moving an object in the direction of an applied force. Work in chemistry would be the energy needed
to overcome attractive forces (inter- & intramolecular forces).
Ex. lifting a book against gravity or moving electrons against nuclear force (Zeff)
- Work = Force x distance [ W = F x d ]
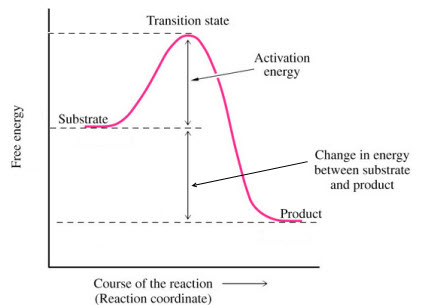
3. A Chemical reaction represents a change in potential energy:
Energy(Reactants)
![]() Energy(Transition State)
Energy(Transition State) ![]() Energy(Products)
Energy(Products)
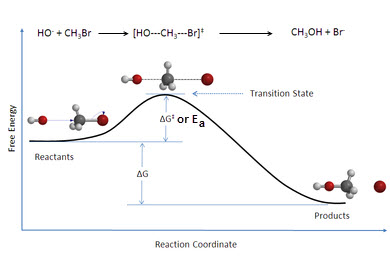
B. Kinetic Molecular Theory- Molecules require a certain amount of energy and correct orientation in order to react.
1. Activation Energy (Ea)- The minimum amount of energy that reactants require in order to react. This represents the
transition state of a reaction.
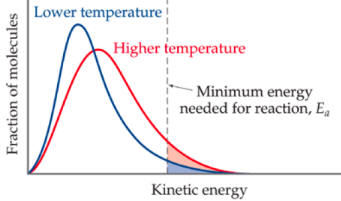
Svante Arrhenius proposed this concept based on the kinetic energy of the particles. The Maxwell-Boltzman distribution
curve potentially identifies the particles (with highest K.E.) that can obtain this activation energy. The energy needed to
overcome inter- & intramolecular attractions.
C. Free Energy- The total energy difference between reactants and products
1. First Law of Thermodynamics- (Law of Conservation of Energy). Energy can neither be created nor destroyed, but can change forms.
The total energy (system & surroundings) must be conserved.
2. Enthalpy Changes (DH): The heat changes associated with chemical & physical changes at constant pressures. Results from changes in potential energy of the
reactants and products.
- We typically ignore the PDV work due to very small changes in system volume
calorie- a unit of heat energy. The energy needed to raise 1 g of water by 1 Co.
4.184 J = 1 cal
a. Enthalpies of Reaction- The change in heat energy associated with a chemical reaction.
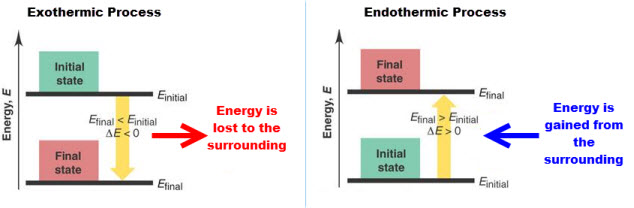
i. Exothermic (-DH)- Energy is released (system
![]() surroundings); PE(reactants) > PE(products)
surroundings); PE(reactants) > PE(products)
Exothermic reaction tend to be spontaneous because they reduce the energy of a system.
Exothermic processes are typically irreversible.
ii. Endothermic (+DH)- Heat is absorbed (surroundings
![]() system); PE(reactants) < PE(products)
system); PE(reactants) < PE(products)
iii. Thermochemical equations: Chemical equations that also include the enthalphy change
Ex. 2H2(g) + O2(g) --> 2H2O(g) + 483.6 kJ
This is exothermic because energy is a product so DH = -483.6 kJ.
iv. Characteristics
1. Enthalpy changes are dependent upon the mass of the reactants (extensive property)
2. Enthalpy changes are numerically equivalent but opposite in sign for the reverse reactions
3. Enthalpy changes are dependent upon the state of the reactants and products.
b. Hess's Law- Chemical reactions can be considered to exist in individual steps and the total enthalpy change is the sum of the enthalpies for each step.
Practice: Hess's Law
c. Enthalpies of Formation (DHf)- The enthalpy change associated with the production of 1 mol of a compound from its constituent elements in their standard state.
i. Standard enthalpy of formation (DHfo)- The enthalpy of formation at Standard State (1atm & 25 oC)
The DHfo for any element in its most stable form is zero.
ii. For any reaction, the enthalpy of reaction is the stoichiometric sum of the individual enthalpies of formation
|
DHorxn = DH1 + DH2 + DH3 + . . . . |
where DH1 = n1(DHfo) ; n1 is the stoichiometric equivalence for compound 1.
Re: DHfo for a reactant is a negative value because we are not forming it, but breaking it down.
iii. Born-Haber Cycle- An indirect process for calculating the lattice energy of an ionic salt by using Hess's Law, enthalpy of formations and energy diagrams.

-lattice energy is a measure of strength of attraction of ions in an ionic salt. The attraction of ions is an exothermic process which will yield a -DH value.
|
|
|
Practice: Born-Haber Cycle
3. Entropy: The measure of disorder associated with a system. Entropic changes are temperature dependent (TDS)
a. Entropy Changes (DS): State functions that describe the energy changes associated with a change in order.
|
DS = q / T (@ constant Temp) |
where q is the energy that is transferred into the system during a spontaneous, reversible process.
i. Enthalpy changes changes with phase changes (DHvap & DHfus)
ii. Enthalpy changes associated with chemical changes (DHfo)
Entropy changes also occur during isothermal expansions/contractions of gases
|
DS = nR ln(V2/V1) (for ideal gases at constant Temp) |
Changes that increase entropy (+DS) tend to be spontaneous.
b. 2nd Law of Thermodynamics- For a spontaneous change in the universe to take place at constant temperature and pressure,
the entropy of the universe must increase.
-This applies to any closed system where DH is zero.
c. The entropy of a pure crystalline element or compounds at absolute zero is zero.
-At absolute zero, an object has zero kinetic energy. This means there is no motion and therefore no stored energy.
The degrees of freedom for the particles is zero.
d. Entropy changes occur when:
i. Liquids or solutions a formed from solids
ii. Gases are formed from either solids or liquids
iii. The moles of gas molecules increases during a chemical reaction
iv. The temperature of a substance increases
Diagram: Comparing phase changes and entropy.
4. Free Energy: The total energy change associated with a system. (Enthalpy & Entropy)
Gibbs-Helmholtz Free Energy:
|
DG = DH - TDS (@constant temperature) |
a. Endergonic: (+DG): The total energy is increased in the system.
b. Exergonic: (-DG): The total is decreased from the system.
c. Spontaneous processes requires a release of free energy
i. If DG is negative, the reaction is spontaneous in the forward direction
ii. If DG is zero, the reaction is at equilibrium
iii. If DG is positive, the reaction requires an input of energy from the surroundings in order for the reaction to proceed.
The reverse reaction will be spontaneous.
|
|
+DH |
-DH |
|
+DS |
Spontaneous when TDS > DH (high temps) |
Always spontaneous |
|
-DS |
Never spontaneous |
Spontaneous when -DH < -TDS (low temps) |