
Unit 6- Redox Reactions & Electrochemistry
The movement of electrons between chemical species is the heart of chemical reactions.
A. Redox Reactions
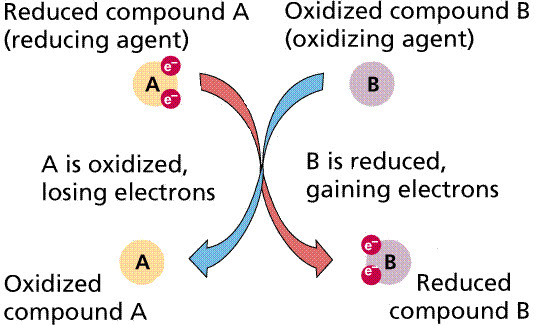
Reduction- chemical process where electrons are GAINED
Oxidation - chemical process where electrons are LOST.
- LEO goes GER (Lose Electrons is Oxidation / Gain Electrons is Reduction)

Redox (reduction/oxidation) reactions
- Are coupled chemical processes where electrons are transferred from one substance to another
-oxidizing agent (oxidant) - the chemical species which oxidizes another (is reduced itself)
-reducing agent (reductant)- the chemical species which reduces another (is oxidized itself)
B. Oxidation states (numbers)
The oxidation state of an atom (or bonded atoms) reflects the degree of oxidation of the chemical substance, usually written as an integer value with positive, negative or no charge.
Atoms that gain or lose electrons become ions. The charge of the ion is a true representation of its oxidation state. The formation of covalent bonding may not produce ions but can change the distribution of electrons around the atoms which can still be represented by an oxidation state.

C. Assigning oxidation numbers
D. Writing & balancing redox reactions
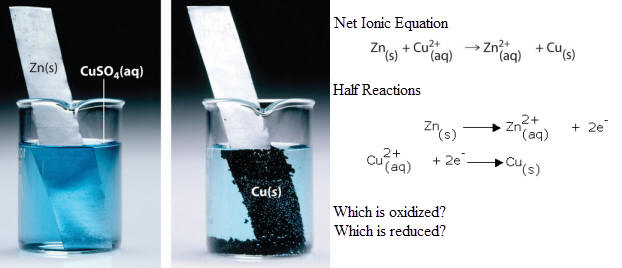
Half-reactions show the individual reduction and oxidation reactions as separate equations. These are written as ionic equations (showing charges)
Balancing Redox Reactions in Acidic Solution
|
1. Assign oxidation states to each element and examination of the oxidation states in order to indicate which substance is oxidized and which is reduced
Alcohols are readily oxidized by acidic solutions of dichromate ions (chromium trixoide in sulfuric acid). 2. Divide the equation into two half-reactions; an oxidation half-reaction and reduction half- reaction by grouping appropriate species. (red.) (Cr2O7)-2 ----> Cr+3 (ox.) C2H6O ----> C2H4O 3. Balance the non-hydrogen and non-oxygen atoms in both half-reactions. (red.) (Cr2O7)-2 ----> 2 Cr+3 (ox.) C2H6O ----> C2H4O 4. Balance oxygen atoms using water (H2O) molecules. Use 1 molecule of water for each oxygen atom that needs to be balanced. (red.) (Cr2O7)-2 ----> 2 Cr+3 + 7 H2O (ox.) C2H6O ----> C2H4O 5. Balance hydrogen atoms using hydrogen ions (H+). Use one (1) H+ ion for every hydrogen atom that needs to balanced. (red.) 14 H+ + (Cr2O7)-2 ---> 2 Cr+3 + 7 H2O (ox.) C2H6O ---> C2H4O + 2 H+ 6. Balance the positive and negative charges by adding electrons (e-). Each electron has a charge equal to (-1). To determine the number of electrons required, find the net charge of each side the equation.
7. Multiply each half-reaction by the smallest whole number that is required to equalize the number of electrons gained by reduction with the number of electrons produced by oxidation. (ox.) 3 C2H6O ---> 3 C2H4O + 6 H+ + 6e- 8. Add the two half reactions and cancel species which are on both sides of the arrow.
|
Balancing Redox Reactions in Basic Solutions
|
The active ingredient in bleach is the hypochlorite (OCl-) ion. This ion is a powerful oxidizing agent which oxidizes many substances under basic conditions. A typical reaction is its behavior with iodide (I-) ions as shown below in net ionic form.
Balancing redox equations in basic solutions is identical to that of acidic solutions except for the last few steps as shown below. 1. Divide the equation into two half-reactions; an oxidation half-reaction and reduction-reaction by grouping appropriate species.
2. Balance non-hydrogen and non-oxygen atoms in both half-reactions.
The iodine and chlorine are alredy balancedb 3. Balance the oxygen atoms using water molecules.
The oxygen is the reduction step is already balanced 4. Balance any hydrogen atoms by using an (H+) for each hydrogen atom 5. Add electrons (e-) to equalize the net charge on both sides of the equation. Note; each electron (e-) represents a charge of (-1).
6. Multiply each equation by an appropriate small whole number in order to equalize the number of electrons lost with the number of electrons gained (ox) 2 I- ----> I2 + 2e- (red) 2e- + 2 H+ + ClO- ----> Cl- + H2O 7. Add the two equations and subtract "like" terms from both sides of the equation. Subtracting "2e-" from both sides of the equation gives the net equation: 2 e- + 2 I- + 2 H+ + ClO- ----> I2 + Cl- + H2O + 2e-
8. To indicate the fact that the reaction takes place in a basic solution, add one (OH-) unit for every (H+) present in the equation. The OH- ions must be added to both sides of the equation as shown below. 2 OH- + 2 I- + 2 H+ + OCl- -----> I2 + Cl- + H2O + 2 OH- 9. Then, on that side of the equation which contains both (OH-) and (H+) ions, combine them to form H2O. Note, combining the 2 OH- with the 2 H+ ions above gives 2 HOH or 2 H2O molecules as written below. 2 H2O + 2 I- + OCl- ----> I2 + Cl- + H2O + 2 OH- 10. Simplify the equation by subtracting out water molecules, to obtain the final, balanced equation.
Note that both the atoms and charges are equal on both sides of the equation, and the presence of hydroxide ions (OH-) indicates that the reaction occurs in basic solution. |
E. Voltaic cells (Galvanic cells)
Voltaic cells are electrochemical cells that harvest energy from redox reactions. The spontaneous redox processes move electrons through an external path instead of passing directly between redox partners.
Components/characteristics:
cells- aqueous solutions of different salt composition or concentration
external circuit- provides a path for electron to move which always move from the anode to the cathode
electrodes- solid metals that are used as redox partners which are connected to an external circuit
-cathode-- reduction, higher electronegativity, increases in mass, positive electrode (e- flow towards)
-anode-- oxidation, lower electronegativity, decreases in mass, negative electrode (e- flow away from)
salt bridge- a porous connective apparatus which allows for the movement of ions between the cells. This provides a way to keep neutral solutions.
-anions migrate toward the anode & cations migrate toward the cathode
Video: Constructing a Voltaic Cell
Resource: Activity Series of metals
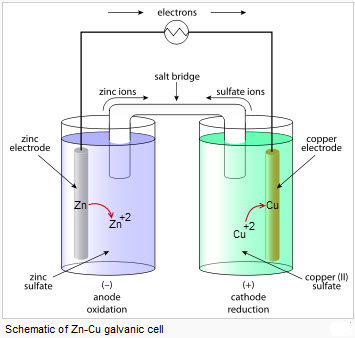
Image: Voltaic Cell
Khan Academy: Galvanic Cell
Electromotive force or cell potential (Emf or Ecell) measures the potential energy difference of electrons between the two redox partners (electrodes).
-electrons tend to spontaneously move to a lower energy state --> lower electronegative metal to a higher electronegative metal (e.g. zinc to copper)
Each electrode has a specific reduction potential based on the metal cations (or nonmetal in solution) ability to gain electrons. The greater the ability an atom/anion has for gaining eletrons the greater its reduction potential.
Emf is measured in volts.
A volt is the potential energy difference associated with 1 coulomb of charge imparting 1 joule of energy.
| 1 coulomb (C) | 6.85 x 1018 electrons | |
| 1 electron (e-) | 1.60 x 10-19 C | |
| 1 volt (V) | 1 Joule / 1 Coulomb ( J/C) | |
| 1 Joule (J) | 1 kg m2 / s2 | |
| 1 Coulomb | 1 ampere x 1 second (A·s) |
Resource: Electrical Potential

table: Standard Reduction Potentials
All reduction potentials are standardized to hydrogen ions gaining electrons, this is called the standard hydrogen electrode potential
Resource: Finding standard reduction potentials

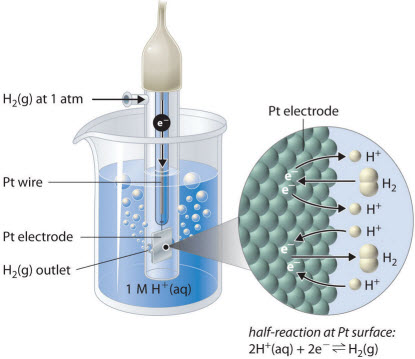
Standard conditions for relative comparison are defined as 1M concentration for all solutions, ambient temperature (298.15 K) & 1 atm pressure.
The standard reduction potential for any material is identified as Eo and the standard cell potential is Eocell
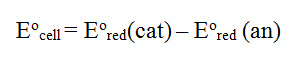
Important points
1. All half-reaction potentials are written as reduction reactions
2. Calculating cell potentials are not stoichiometrically significant. We don't consider coefficients in calculations
3. Don't change signs of the standard reduction potentials. The equation is compensating for the oxidation reactions (minus instead of plus--remember Hess's Law)
4. The more positive the value of Eored, the greater the driving force for reduction. Cathodes are defined by the large Eored.
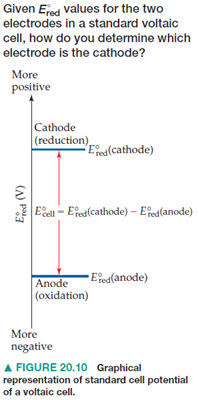 |
A more positive Eored means a substance is more likely to be reduced (oxidizing agent) and the more negative the Eored means a substance is more likley to be oxidized (reducing agent). |
G. Free energy in redox reactions & spontaneity
The free energy associated with a redox process is found by the equation:
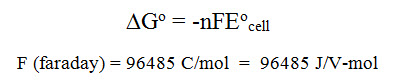
A spontaneous redox process will occur when Eored is positive and the free energy is negative (exergonic)
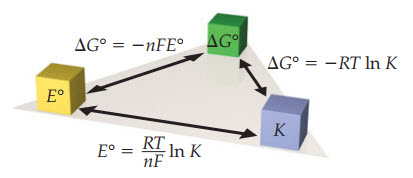 |
Spontaneous process that lead to product formation have the
following: 1. + Eo 2. -DGo 3. K > 1 |
H. Cell Emf under nonstandard conditions
The Nernst equation allows us to calculate the cell potential when concentrations are not 1M, when temperature is not 298 K and pressures are not 1 atm.
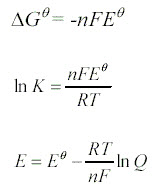
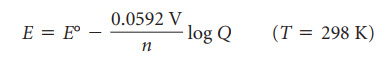
Remember that Q is the reaction quotient, the ratio of product concentrations to reactant concentrations
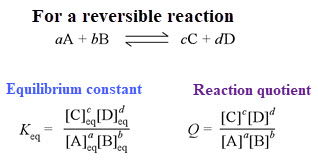
Video: Solving problems with Nernst equation
I. Electrolysis : Khan Academy Video
This is essentially a voltaic cell in reverse. An outside energy source reverses the redox process, driving electrons from the reduced species back to the oxidized species.

Electroplating metals with other metals is an application of electrolytic cells.
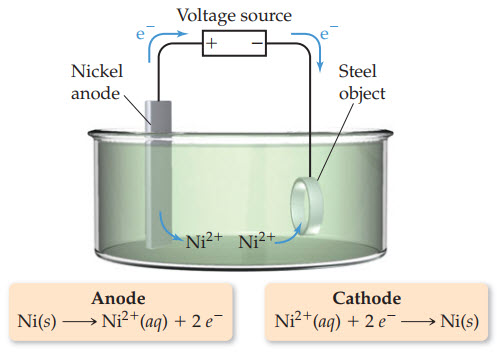
To calculate the quantity of metal added while electroplating, we need current and time.
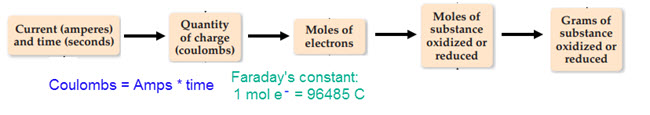
Resource: Solving electrolysis problems
Video: Solving electrolysis problems
Image: Electrochemistry flow chart