The boiling point of a substance is the temperature at which it changes state
from liquid to gas throughout the bulk of the liquid. At the boiling point
molecules anywhere in the liquid may be vaporized.
The boiling point at normal atmospheric pressure (1
atm, 14.7
psi, 101.325 kPa, 760 torr, 760 mmHg, 29.92 inHg1.01325 bar ) for some
common fluids and gases can be found from the table below:
|
Structure |
Product |
Boiling Point at Atmospheric
Pressure(oC) |
Product |
Boiling Point at Atmospheric
Pressure(oC) |
Structure |
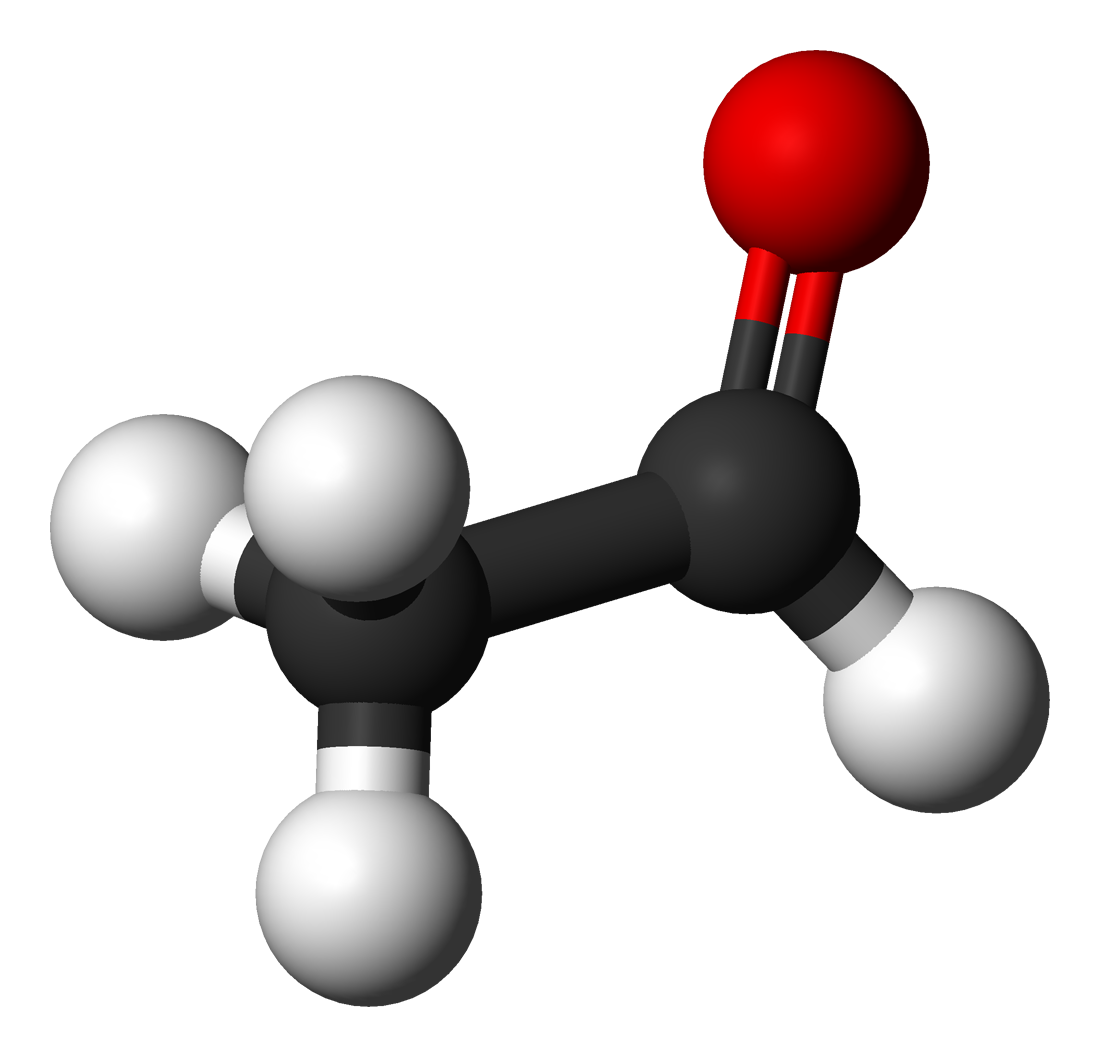 |
Acetaldehyde CH3CHO |
20.8 |
Glycerine |
290 |
 |
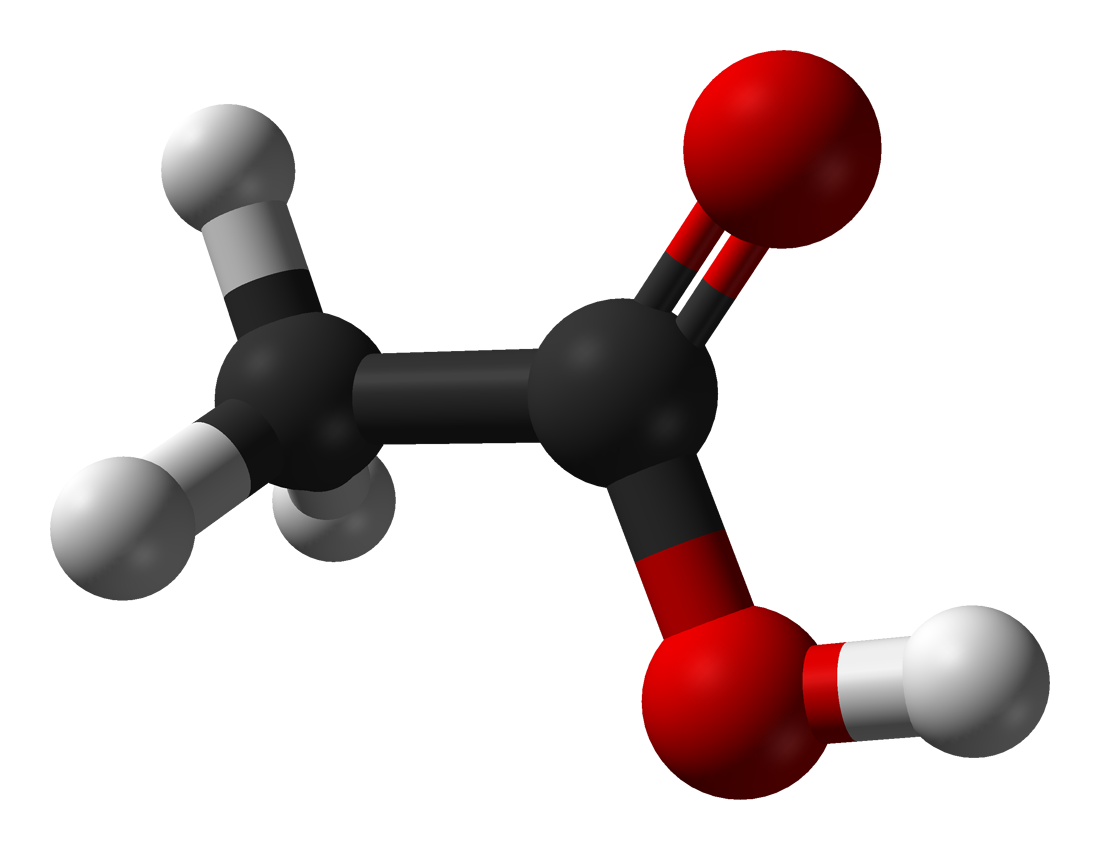 |
Acetic acid |
118 |
Helium |
-269 |
|
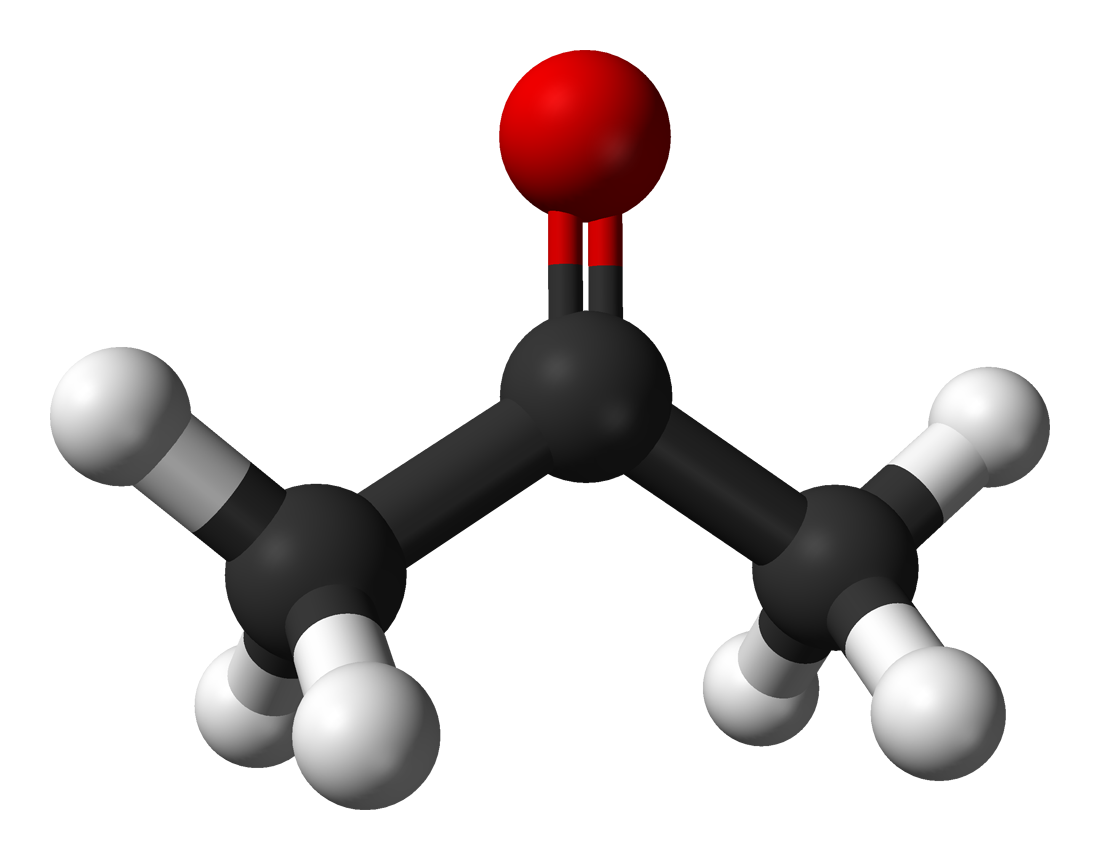 |
Acetone CH3COCH3 |
50.5 |
Heptane-n |
98.4 |
 |
 |
Acetylene |
-84 |
Hexane-n |
68.7 |
 |
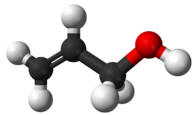 |
Alcohol - allyl (2-propenol) |
97.2 |
Hydrogen |
-253 |
 |
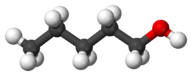 |
Alcohol - butyl-n |
117 |
Iodine |
184.3 |
 |
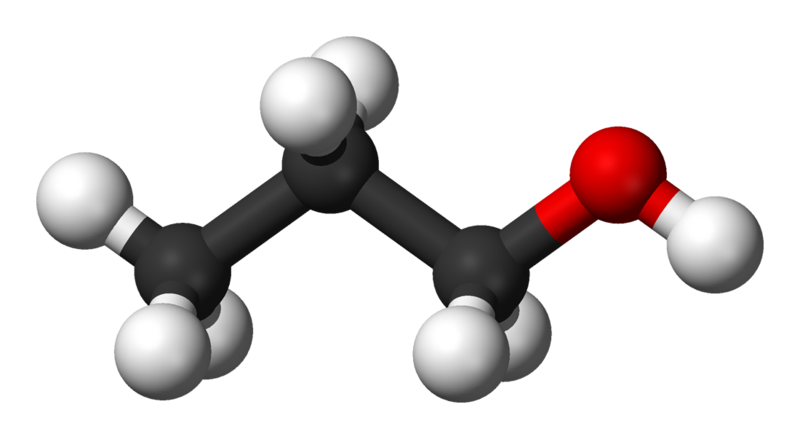 |
Alcohol - propyl |
97.5 |
Isopropyl Alchol |
80.3 |
 |
 |
Ammonia |
-35.5 |
Kerosene (dodecane) |
150 - 300 |
 |
 |
Aniline (C6H5NH2) |
184.4 |
Linseed Oil |
287 |
 |
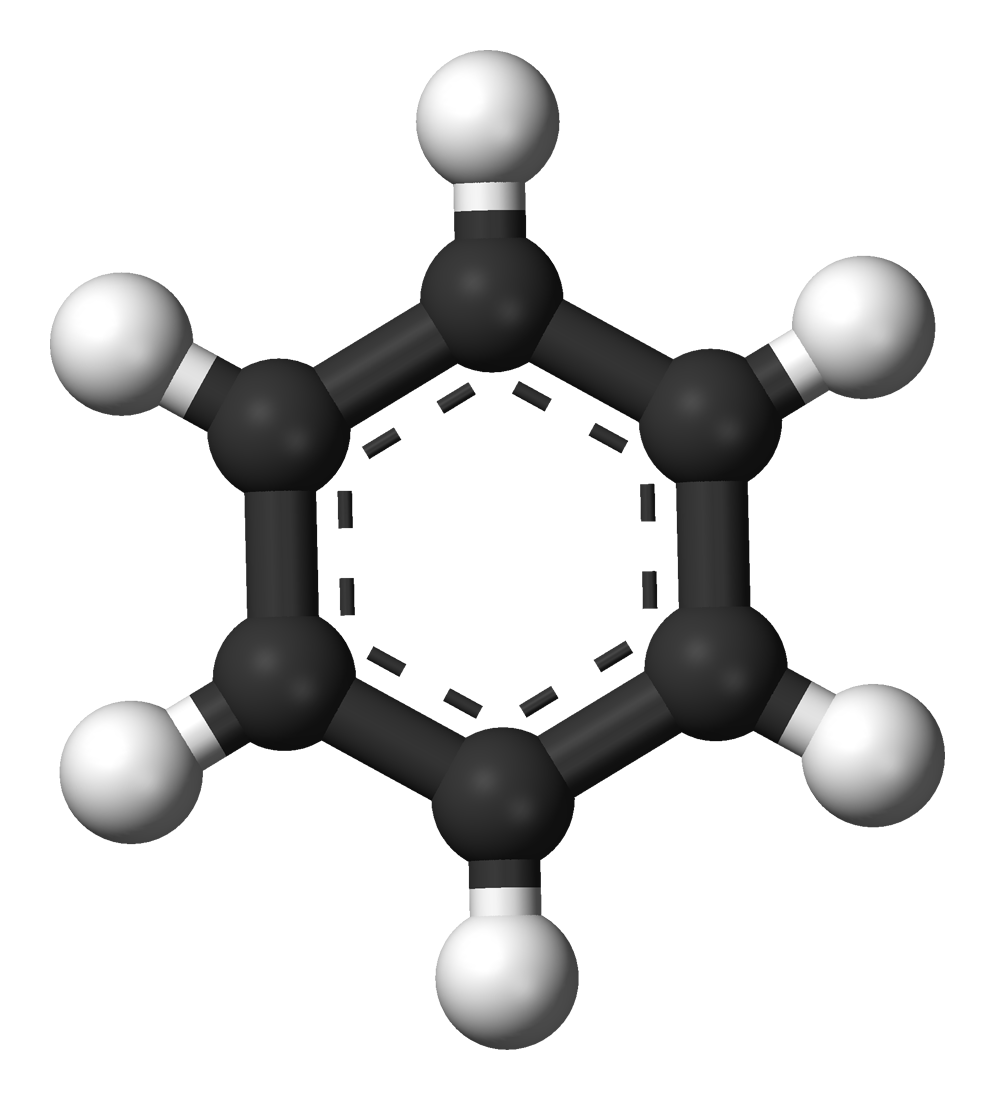 |
Benzene (Benzol) C6H6 |
80.4 |
Mercury |
356.9 |
|
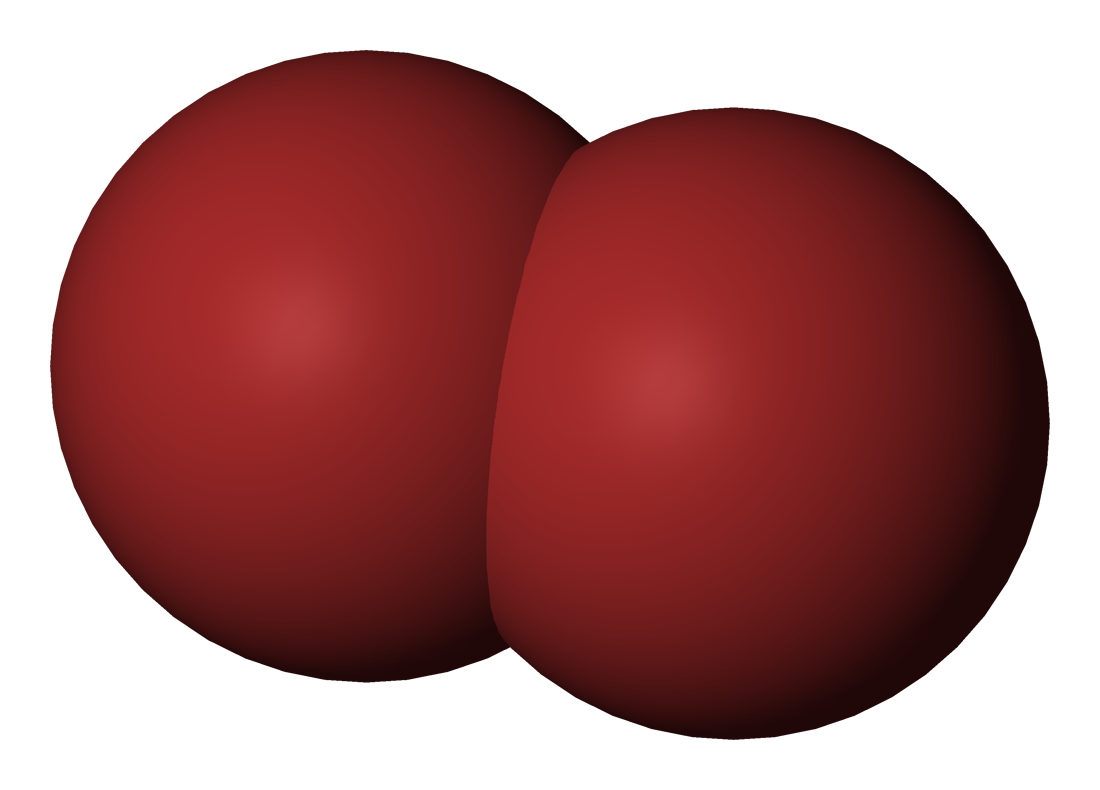 |
Bromine |
58.8 |
Methanol (methyl alcohol, wood alcohol) |
66 |
 |
 |
Bromobenzene |
156.0 |
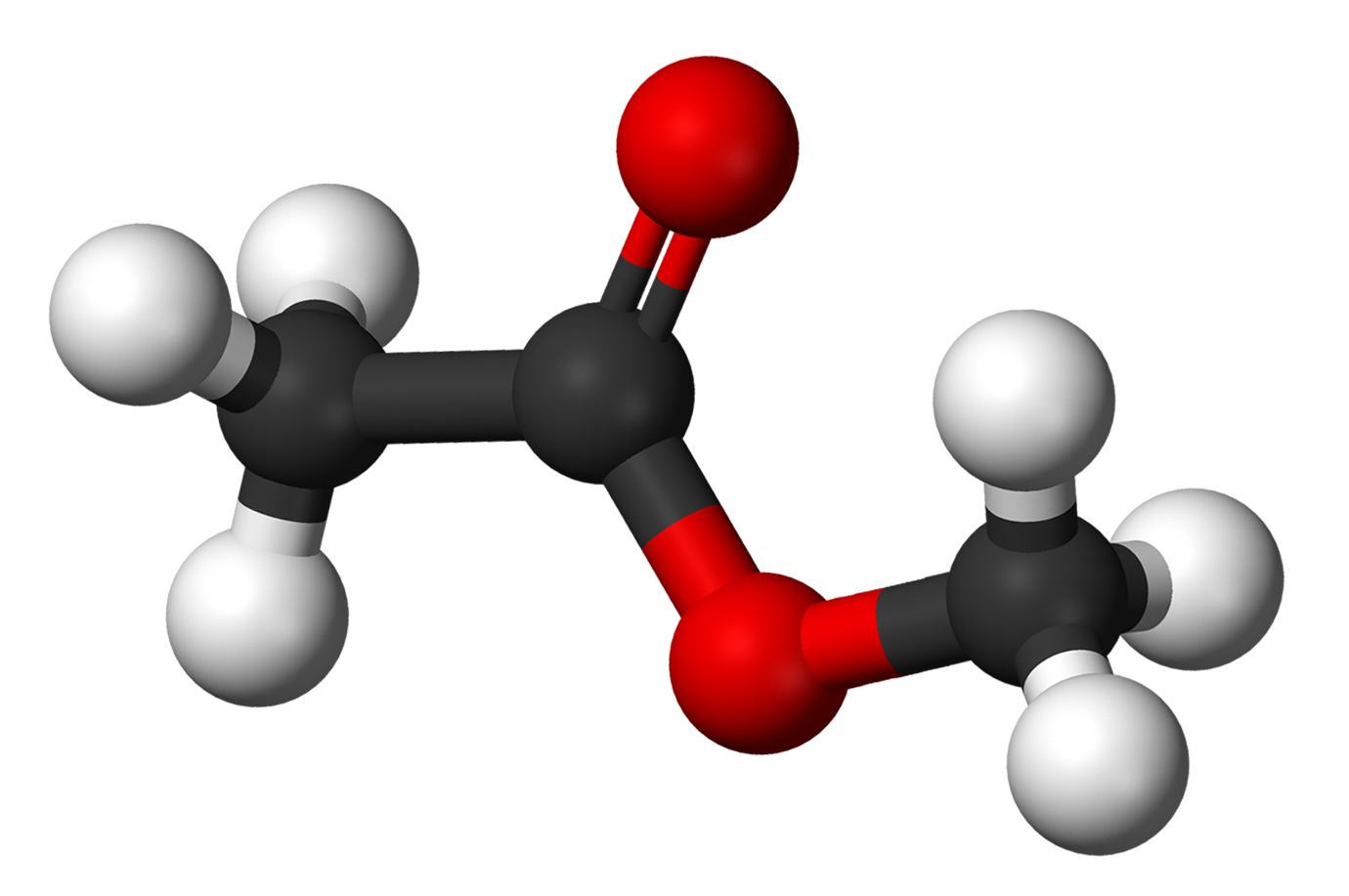
Methyl acetate |
57.2 |
 |
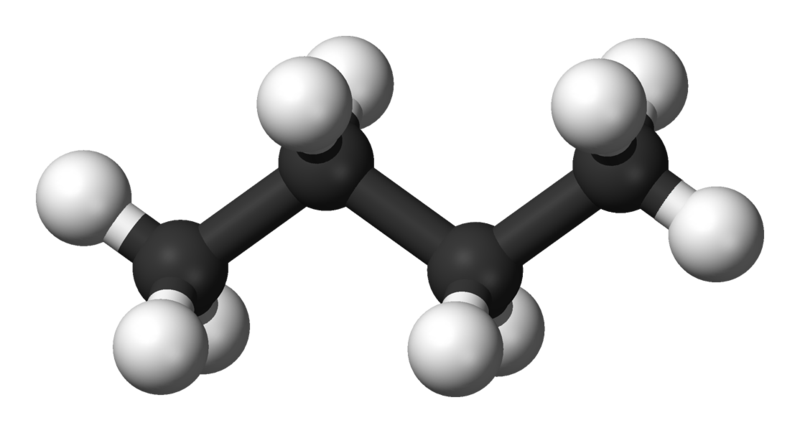 |
Butane-n |
-0.5 |
Methylene Chloride(CH2Cl2,
dichloromethane) |
39.8 |
 |
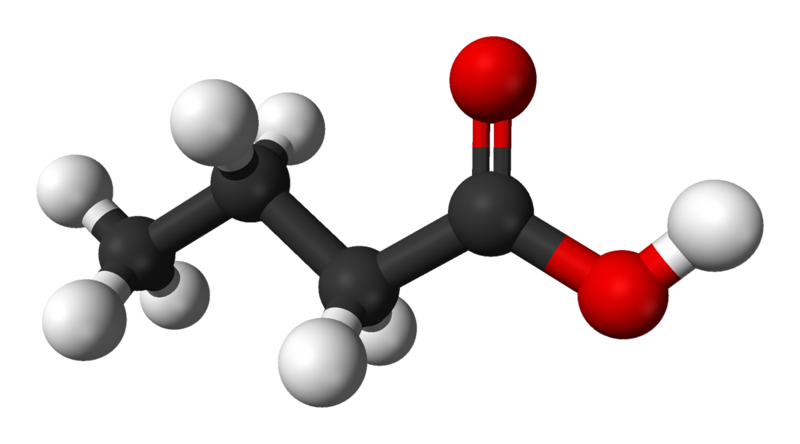 |
Butyric acid |
162.5 |
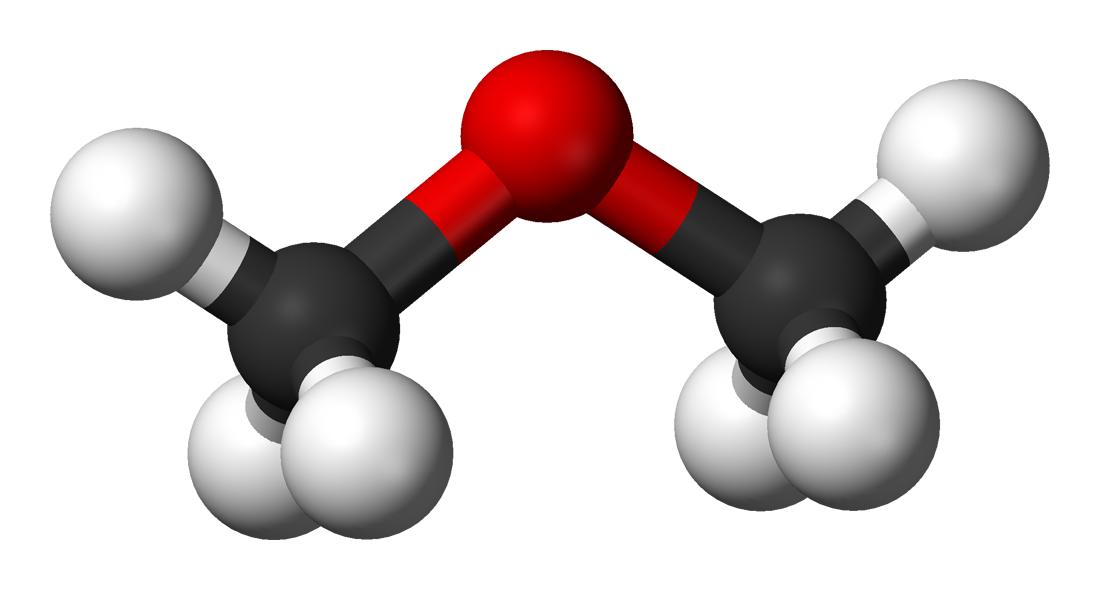
Methyl Ether (C2H6O) |
-25 |
 |
 |
Carbon disulfide (CS2) |
47.8 |
Methyl iodide |
42.6 |
 |
 |
Carbon dioxide |
-57 |
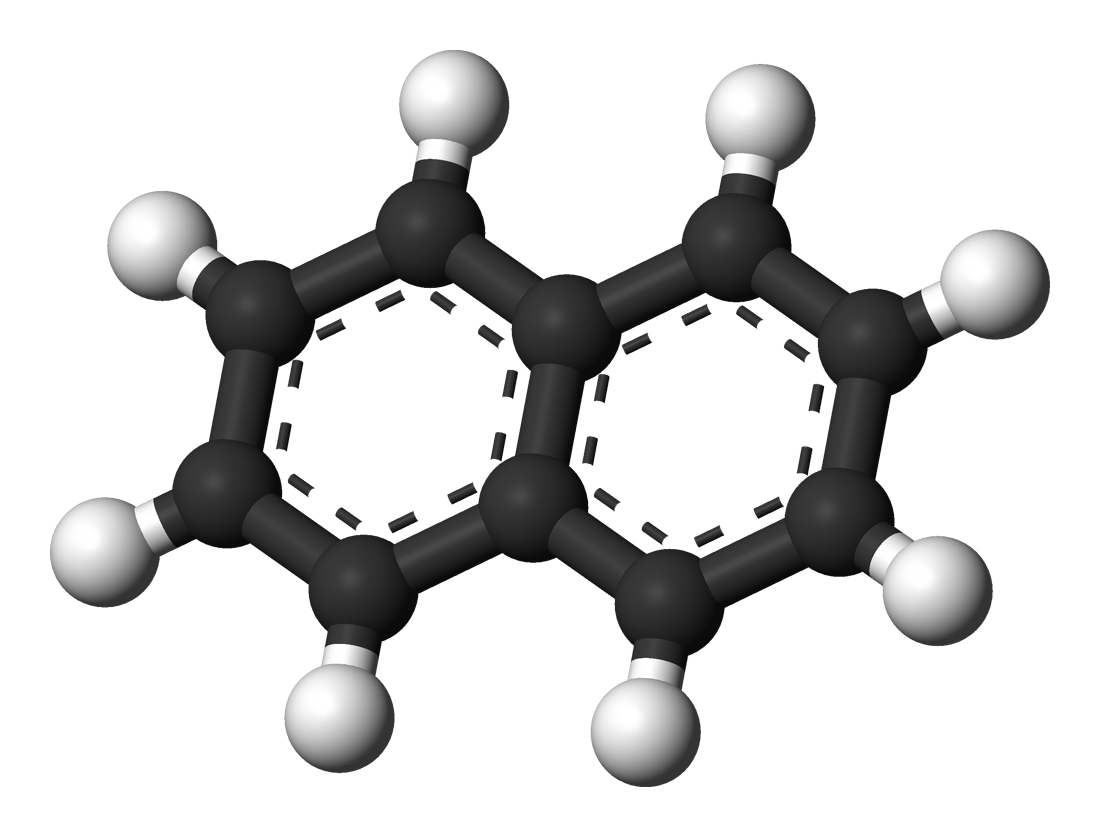
Naphthalene (Napthaline) |
217.9 |
 |
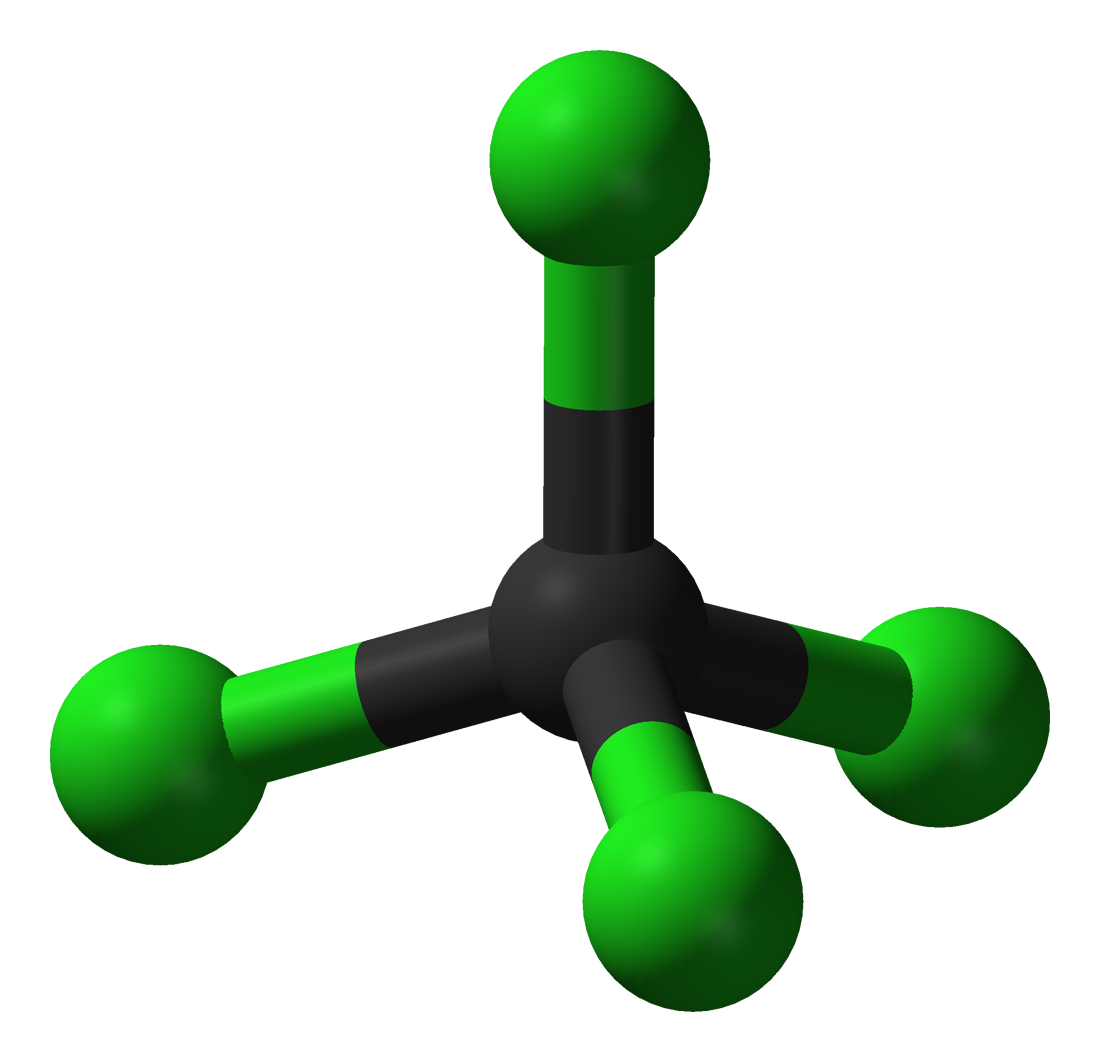 |
Carbon tetrachloride CCl4 |
76.7 |
Nitrobenzene (C6H5NO2) |
210.9 |
 |
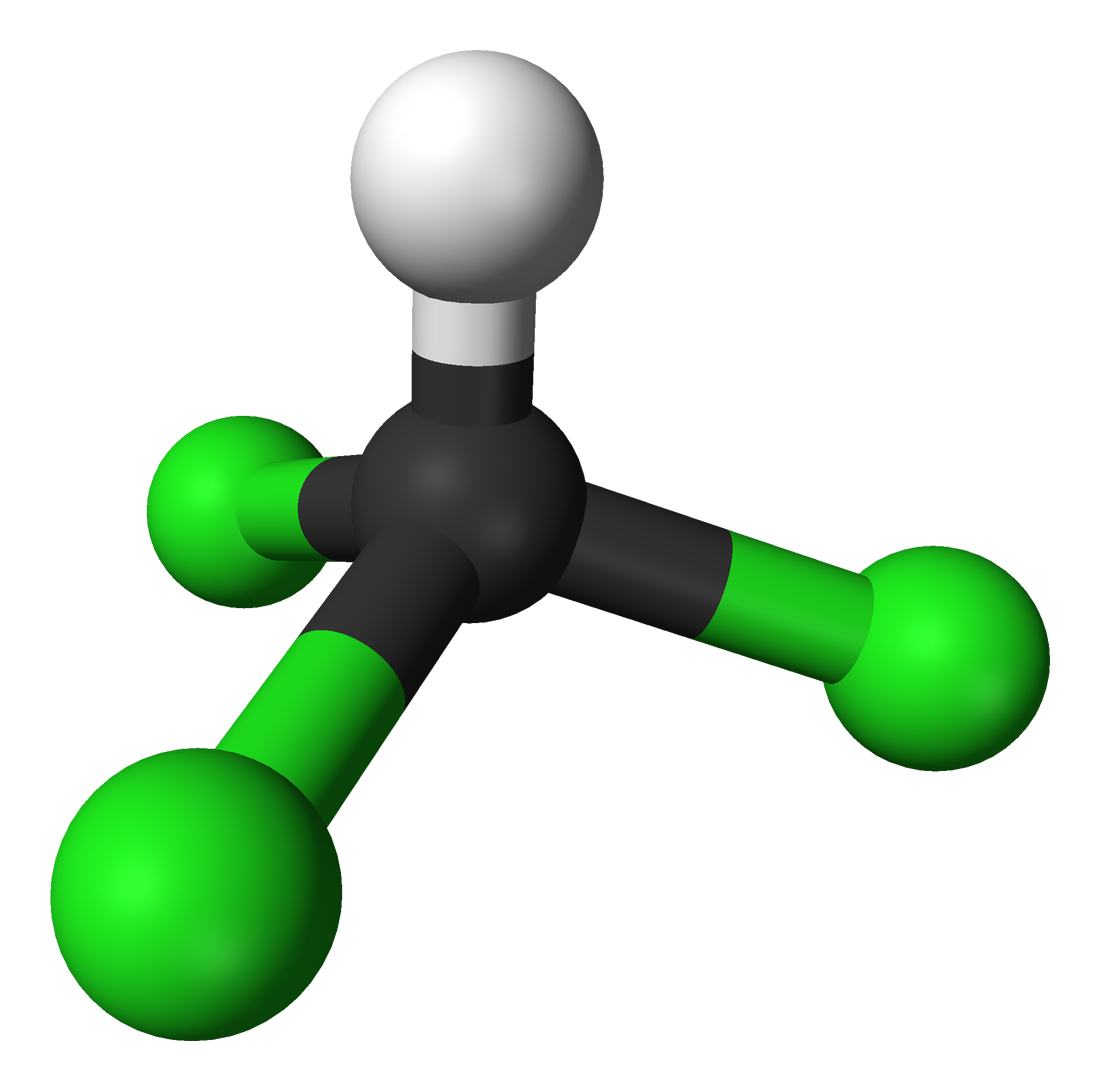 |
Chloroform |
62.2 |
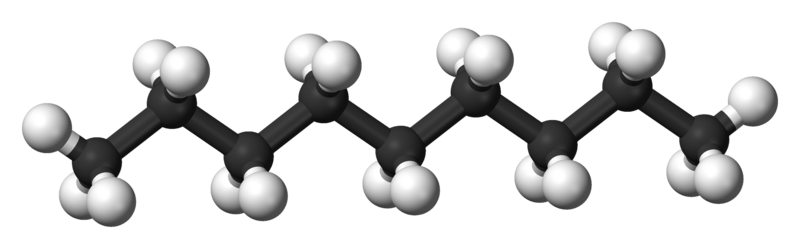
Nonane-n |
150.7 |
 |
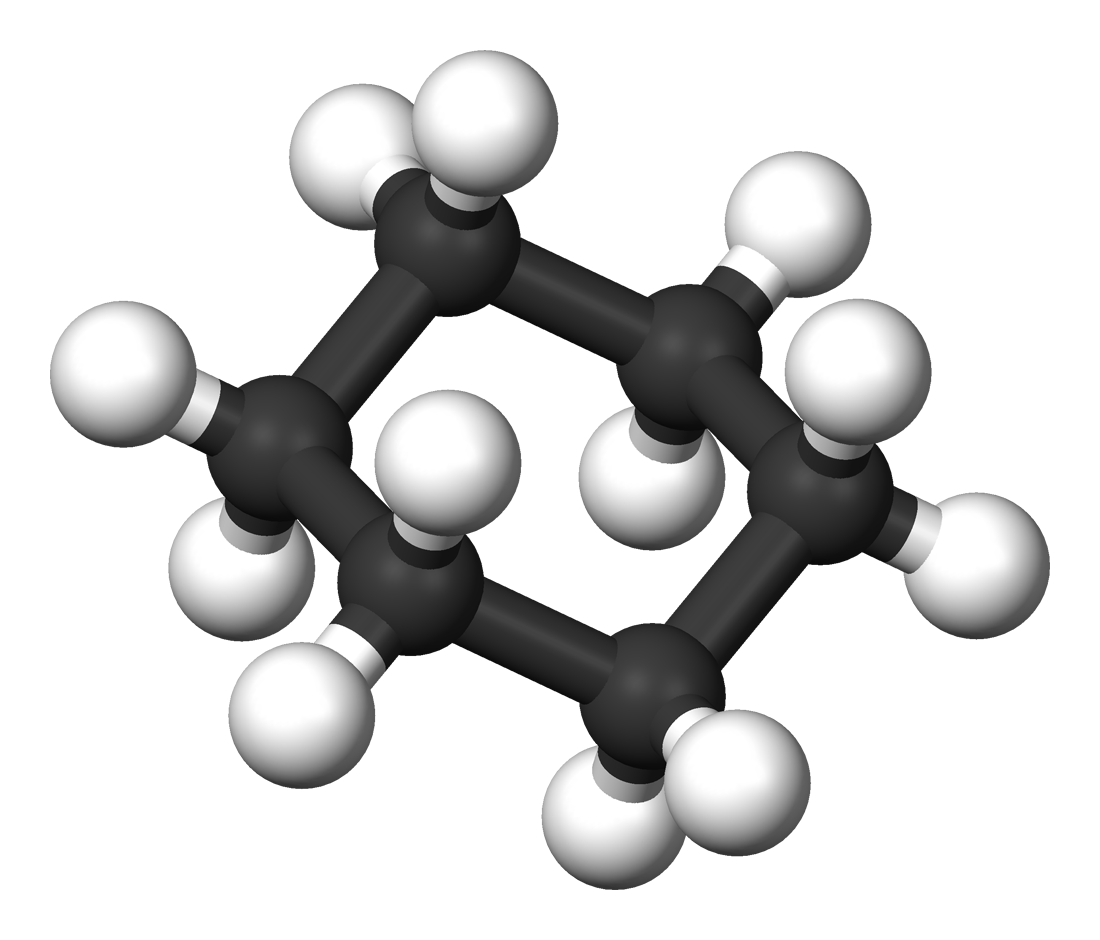 |
Cyclohexane |
80.7 |
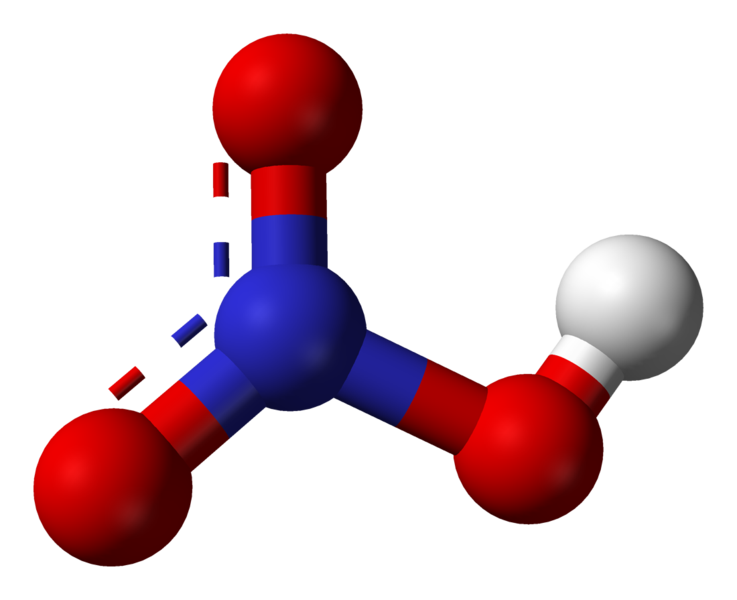
Nitric Acid (anhydrous) |
120 |
 |
 |
Decane-n |
173 |
Nitrogen |
-196 |
 |
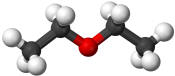 |
Diethyl ether |
34.7 |
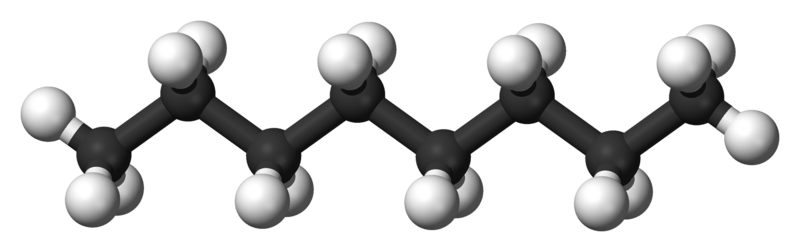
Octane-n |
125.6 |
 |
 |
Ethane C2H6 |
-88 |
Oxygen |
-183 |
 |
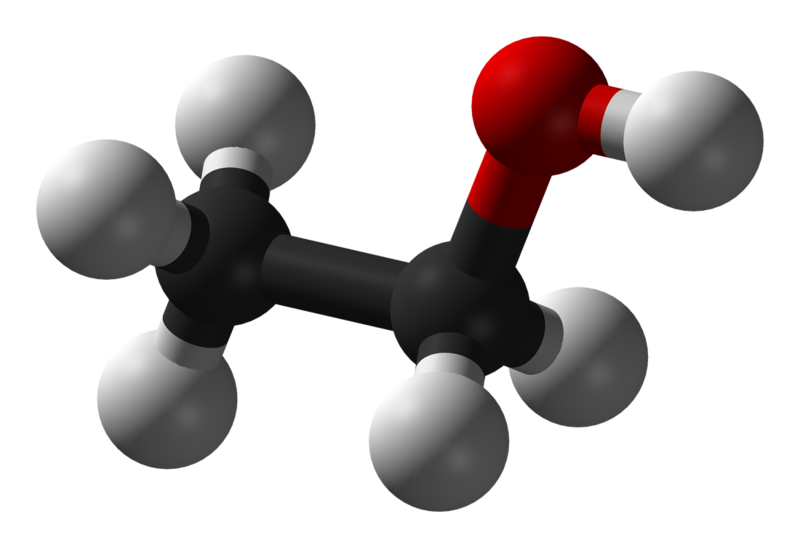 |
Ethanol |
78.4 |
Pentane-n |
36 |
 |
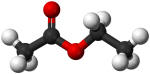 |
Ethyl acetate CH3COOC2H3 |
77.2 |
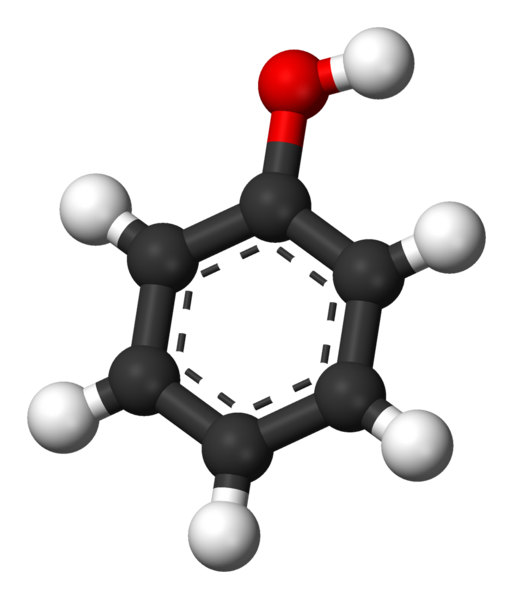
Phenol (C6H5OH) |
182 |
 |
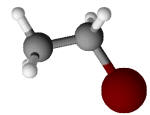 |
Ethyl bromide C2H5Br |
38.4 |
Propane |
-43 |
 |
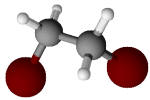 |
Ethylene bromide (C2H4Br2) |
131.7 |
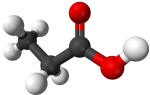
Propionic acid |
141 |
 |
 |
Ethylene Glycol |
197 |
Propene |
-47.7 |
 |
 |
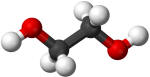
Formic acid |
101.0 |
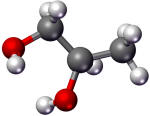
Propylene glycol |
187 |
 |
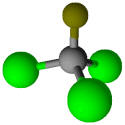 |
Freon refrigerant R-11 (CFCl3) |
23.8 |
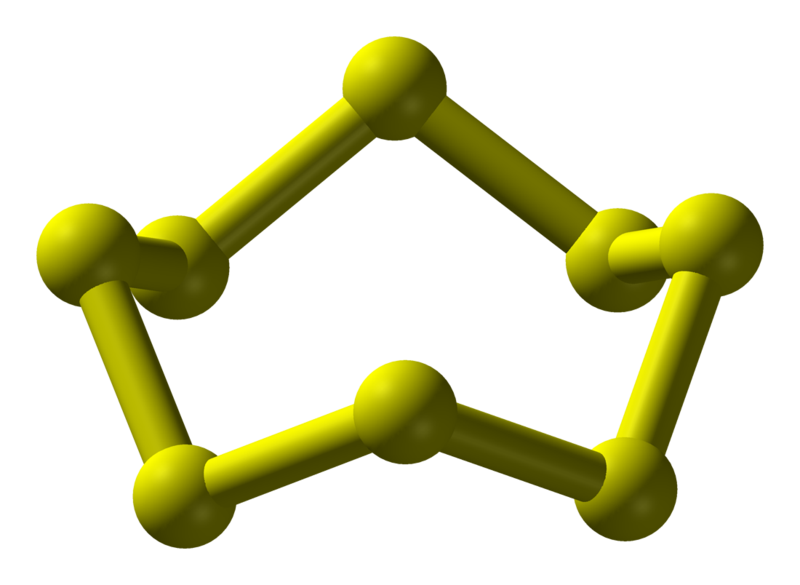
Sulphur |
444.6 |
 |
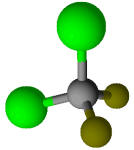 |
Freon refrigerant R-12 (CF2Cl2) |
-29.8 |
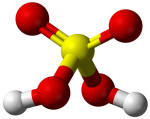
Sulphuric acid |
310 |
 |
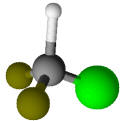 |
Freon refrigerant R-22 |
-41.2 |
Toluene (C6H5CH3) |
110.6 |
 |
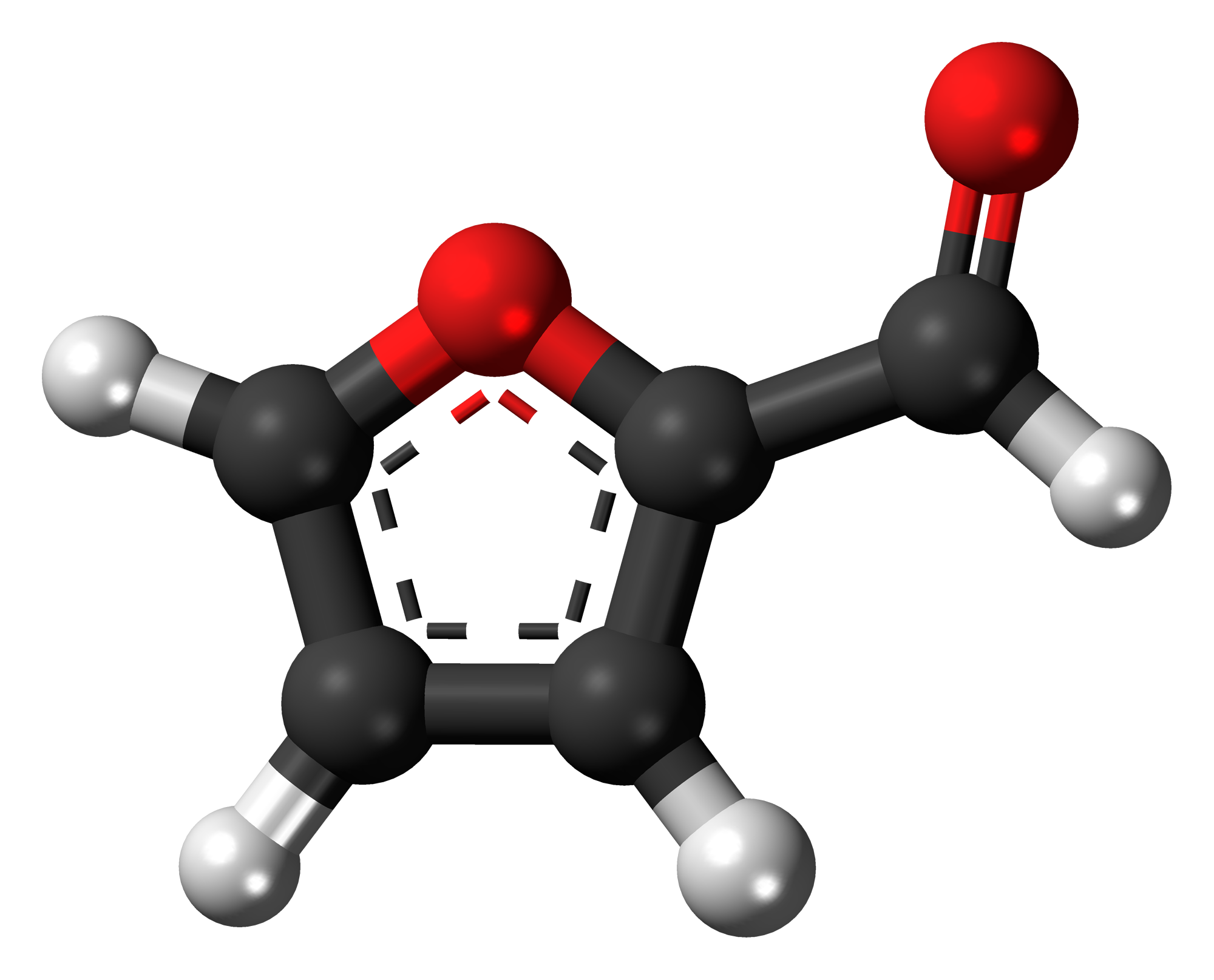 |
Furfurol |
161.7 |
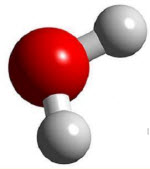
Water |
100 |
 |
- TF
= 1.8*TC+ 32
- TC = (TF - 32)/1.8
- Tk = TC + 273.15



























































