|
II.
Radioactivity
|
|
|
|
|
|
A. Nucleons- The
components of the nucleus. Protons (11p)
and Neutrons (10n)
|
|
|
|
1. atomic number (Z)- the number of protons
|
|
|
|
2. mass number (A)- the number of nucleons in an atom (protons + neutrons)
|
|
|
|
3. isotopes- atoms of the same elements that differ by the number of
neutrons
|
|
|
|
|
a. nuclide- the nucleus of specific isotope of a certain element
|
|
|
|
4. radioisotope- an isotope that contains an unstable nuclide.
|
|
|
|
|
a. radionuclide- the unstable nucleus of a radioisotope
|
|
Video:
Radioactivity-Expect the Unexpected
Resource:
The
story of how radioactivity was discovered --
timeline
Resource:
The
theory behind radioactivity and nuclear stability
|
|
|
B. Atom construction & energies
|
|
|
|
Question: What comprises the helium atom?
|
|
|
|
|
2 protons (@ 1.0073 a.m.u), 2 neutrons (@1.0087 a.m.u.) and 2 electrons (@
0.00055 a.m.u)
|
|
|
|
|
|
Therefore the pieces of the helium atom add up to 4.0331 a.m.u.-- Isotopes
of Helium
|
|
|
|
|
Helium-4 (99.999% of all helium atoms) has a mass of 4.0026.
Where does the other 0.0305 a.m.u. go?
|
|
|
|
1. Mass Defect- the difference in masses between the atom and the sum of the
atom's components.
|
|
|
|
2.
Nuclear Binding Energy- the energy associated with the mass defect
defined by Einstein's equation (E = mc2). It is the energy
associated with nucleus construction.
|
|
|
|
|
|
|
|
|
|
C.
Types of Radioactive particles
|
|
|
|
- All radioactive decay reactions
can be thought of an unstable parent nuclide decaying into a daughter
nuclide.
|
|
|
|
1. Alpha Particles. High speed
"helium nuclei". written as 42He or 42a.
|
|
|
|
|
Associated with heavy isotope
decay (N > 83 and A >= 200)
|
|
|
|
|
|
ex. 21284Po
----> 20882Pb + 42a
|
|
|
|
|
|
Polonium was the first
radioactive element found. Discovered by Marie Curie and her husband
Pierre in 1898.
20882Pb is
the most abundant isotope of lead (~52.4%)
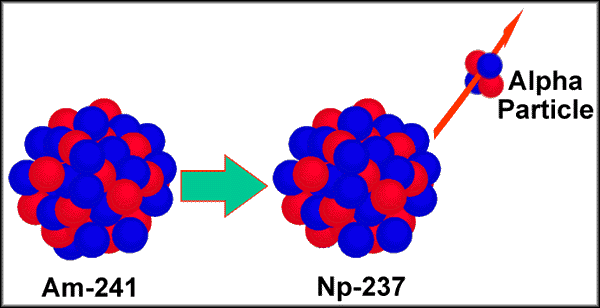
The alpha decay of 241Am
(americium-241) to form 237Np (neptunium-237)
|
|
|
|
2. Beta Particles. High speed
electrons. written as 0-1e- or b
|
|
|
|
|
Associated with neutron
decay. 10n ---> 11p
+ 0-1e-
|
|
|
|
|
|
ex. 9843Tc
---> 9844Ru + 0-1b-
|
|
|
|
|
|
|
Technetium is a radioactive
element that does not occur naturally but instead was artificially prepared
in 1937
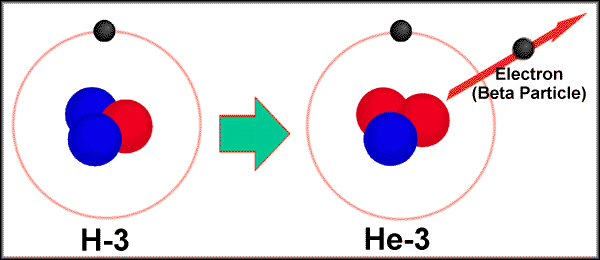
Tritium (31H) decaying into 32He
What is Tritium?
Click
here to read about it.
|
|
|
|
|
|
Rutherford's
discovery of the alpha and beta particles
|
|
|
|
|
|
Exercises
in writing alpha and beta decay equations.
|
|
|
|
3. Gamma radiation.
Electromagnetic radiation with high frequency and high energy. written as g
|
|
|
|
|
Usually accompanies all
radioactive emissions. Represent lost energy.
|
|
|
|
|
|
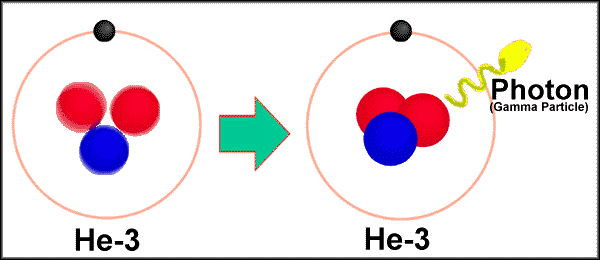
An interesting gamma
emission. The annihilation of an electron and a positron forms gamma
radiation
|
|
|
|
|
|
|
0-1e-
+ 0+1e ----> 2g
( 0.511 MeV or 8.187 x 10-14 J) ( 1 Megaelectron volt
= 1.602189 x 10-13 J)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
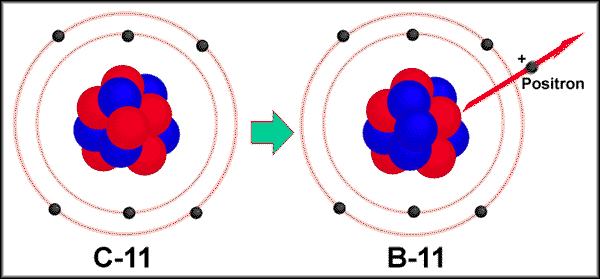
4.
Positron
emission- Particle that is similar to an electron but with a
positive charge. written as 0+1e or b+
|
|
|
|
|
Positron emission essentially
converts a proton into a neutron. 11p
----> 10n + 0+1e
|
|
|
|
|
|
ex. Carbon-11
decay. 116C
---> 115B + 0+1e.
|
|
|
|
|
|
|
Positron
annihilation studies at the University of Bristol, UK.
|
|
|
|
5. Electron capture- The nucleus
captures an n=1 electron.
|
|
|
|
|
Electron capture produces an
effect similar to positron emission, converting a proton to a neutron.11p
+ 0-1e- ----> 10n
|
|
|
|
|
|
ex. 74Be
+ 0-1e- ---> 73Li.

|
|
|
|
|
|
|
|
|
|
|
|
Exercises
in writing positron emission and electron capture equations
|
|
|
|
|
|
|
|
|
|
|
|
D. Patterns of Stability
|
|
|
|
1. Neutron-to-Proton ratios
|
|
|
|
|
Nucleons are held together by
Strong Nuclear Forces.
|
|
|
|
|
The stability of the nucleus is
dependent upon the neutron-to-proton ratio. As Z increases the number of
neutrons needed also increases but not in a linear relationship.
|
|
|
|
|
|
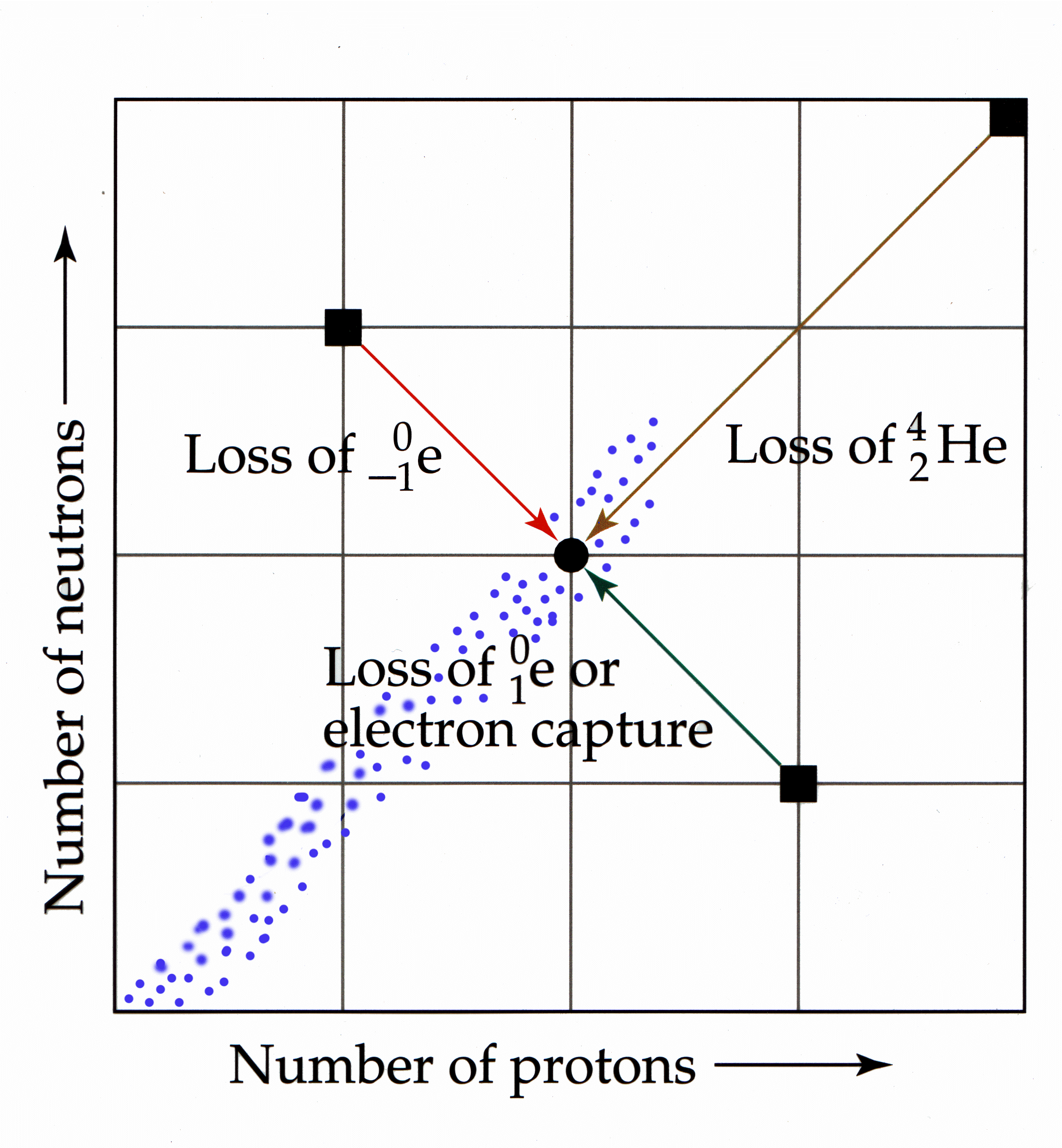
a. Belt of Stability. A
region on a neutron to proton graph that represents stable nuclides
|
|
|
|
|
|
|

|
Notes to Consider:
1. All nuclides with Z>83 are
unstable
2. Z values lower than 20 have
neutron/proton = 1
3. As Z increases above 20, the
neutron/proton ratio increases. ex. 90Zr = 1.25, 120Sn
= 1.4, 200Hg = 1.5
4. Region above the belt
represents excess neutrons
5. Region below the belt
represents excess protons
|
|
|
|
|
|
|
|
|
Resource: Table of
naturally found isotopes and abundances |
|
|
|
|
|
|
Predicting decay based on
neutron-to-proton ratio
|
|
|
|
|
|
|
|
Notes to consider:
1. Red Arrow: Represents high
neutron/proton. Seen as Beta Emission. Increases Z and A remains same
2. Brown Arrow: Represents
Z>83. Alpha particles emitted to reduce both A and Z.
3. Green Arrow: Represents
low neutron/proton. Positron emission or electron capture. Decreases Z
and A remains same
|
|
|
|
|
|
b. Transmutation reactions.
|
|
|
|
|
|
|
Creating unstable nuclei from
stable nuclei through bombardment of neutrons or other nuclei
|
|
|
|
|
|
|
The first transmutation was
performed by Ernest Rutherford in 1919. Bombarding 14N with alpha
particles
|
|
|
|
|
|
|
147N
+ 42a ---> 178O
+ 11p+
Both the oxygen and
hydrogen are stable so there is no further decay.
For transmutation
reactions, the bombarding particle and the product particle are written in
parentheses between the symbols for the reactant and product nuclides.
ex. 147N (a,p)178O
|
|
|
|
|
|
|
|
|
|
|
|
|
c. Nuclear decay series.
Nuclides with Z much larger than 83 cannot decay to stable nuclides with one
emission. This usually requires multiples steps representing multiple
decay emissions. U-238 decays through a series
of alpha and beta emissions before becoming stable.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
An
applet
that demonstrate the decay series of some
transuranium
elements
|
|
|
|
|
|
|
A
historical
perspective of where the names for the transuranium elements were
derived.
|
|
|
|
|
|
|
A
historic
film showing transuranium elements. Hosted by Glenn Seaborg
|
|
|
|
|
|
d. Stability based on nucleon
numbers in an atom's
nucleus.
Magic Numbers.
|
|
|
|
|
|
|
Specific elements demonstrate a
greater stability than others. This is due to nucleon configurations.
|
|
|
|
|
|
|
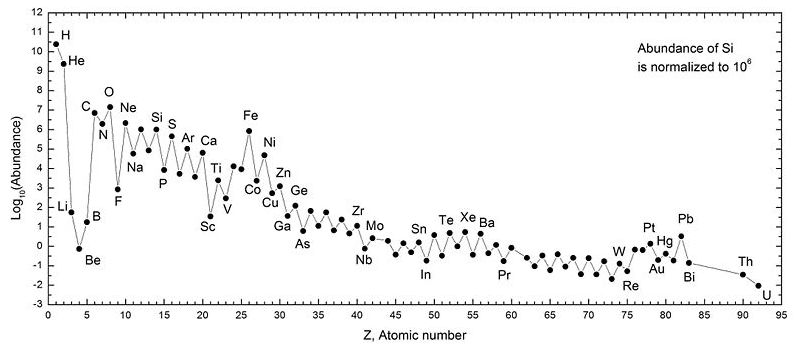
1. Elements with Z = 2, 8, 20,
28, 50, and 82 are typically more stable than others
|
|
|
|
|
|
|
2. Elements with even-numbered Z
and neutrons are generally more stable than odd-numbered nucleons
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
There are
new
elements being made that demonstrate a higher degree of stability than
some.
Island
of Stability
|
|
|
|
|
|
|
News
release regarding the new elements Z = 116 and 118.
|
|
|
|
|
|
|
Video on
Island of Stability |