
resource: The Atom. by Wikipedia
I. A History of the Atomic Theory- "How Small is Small"
A. Democritus- Greek- "atomos" (means: indivisible) "All matter is composed of indivisible units."
B.
John
Dalton- English Physicist (1766-1844)
Dalton's Atomic Theory (Khan Academy)-
1. All elements are composed of atoms and the atom is the smallest piece of that element
2.All atoms of the same element are the same. These atoms then have the same mass.
Law of Definite Proportions ( Law of Constant Composition) - All compounds are composed of specific ratios of elements.
video: Law of Definite Proportions
-This was actually coined by Joseph Proust, which Dalton used to develop his theory
4. Chemical reactions occur when atoms are separated, joined or rearranged.
 |
Atoms of Salt, Water & Iron. The chemical properties were conserved in the structure of the atoms |
II. Composition of the Atom & Historical Atomic Models.
Model: Simplified View of the Subatomic Particles.
Video: How small is the atom- TedEd
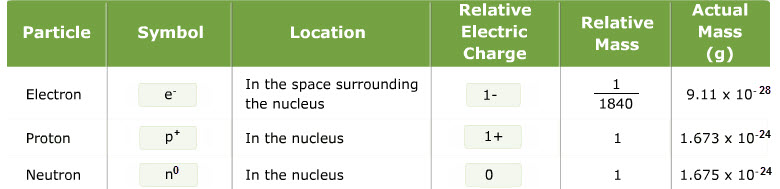
A. Electron- negative particles, very small
1. William Crookes (1832-1919)- Cathode Ray Tube- (Crooke's Tube)
-cathode rays-
a. travel from cathode to anode in straight lines
b. heated metals placed in path of rays
c. deflected by electric & magnetic charges- shows rays are made of negative particles.
2.
J.J.
Thomson (1856-1940)- 1897 England-
Using
Crookes Tube
Thomson's experiment lead to identification of the electron.
Resource: Results of Thomson's experiment.
-measured deflection of electrons in magnetic & electric fields-
-found the ratio of charge to mass (e/m)
-determined the ratio was constant regardless of the gas used.
Thomson's presentation for the Nobel Prize, 1906
Video: The discovery of the electron
Videos: Cathode ray tubes & the electron. Magnetic force on cathode ray tube
3. R.A. Millikan- 1909- using X-ray induced charged oil droplets
-found the charge and mass of electrons- mass = 0.00055 a.m.u (1/1837 amu)
Movie: Oil drop experiment, the charge of an electron
B. Proton- positive particles- more massive than electrons
1. Eugen Goldstein- 1886- Using a canal ray tube
- observes that a cathode-ray tube produces, in addition to the cathode ray, radiation that travels in the opposite direction
away from the anode; these rays are called canal rays because of holes (canals) bored in the cathode; later these will be
found to be ions that have had electrons stripped in producing the cathode ray.
- Ernest Rutherford is now credited with the discovery of the proton particle from alpha bombardments with nitrogen.
Video: modified Crookes Tube
2. Wilhelm Wien- 1898- found charge to mass ratio
-ratio
is smaller than that of the electron but varied with kind of gas
-Hydrogen has smallest ratio- mass of proton = 1.0073 a.m.u
-His studies were paralleled by Thomson & Rutherford and eventually led to mass spectroscopy
C. Neutron- neutral in charge- mass is comparable to proton
1. James Chadwick- (1891-1974)- 1932, finding the neutron
-bombarded beryllium atoms with high velocity alpha particles and emitted uncharged particles:
The reaction of the experiment can
be written in the form:

-unstable when not in atom- decay into proton and electron
- Here is an outline of Chadwick's experiment
D. Elementary Particles- particles that make up the subatomic particles
The Sub-Atomic Zoo. A comprehensive outline of elementary particles
The Particle Adventure- a trip into defining elementary/fundamental particles
A. Nucleus- central dense region containing proton/neutrons.
1. Ernest Rutherford Gold Foil Experiment (1871-1937)- 1911- (more on Rutherford)
-Bombarded gold foil with high velocity alpha particles. Some particles were deflected, scattered, showing a dense
region within atoms containing positive region.
An abstract introducing Rutherford's paper on a - particle scattering.
Video: Discovering the nucleus -- Modern day gold foil experiment
Video: Scattering radioactive particles
Video: Rutherford's experiment
Image: Disproving plum-pudding
2. Henry Moseley- (1887-1915)- 1913- Moseley's Experiment
-Using an X-ray tube (modified cathode ray tube) to identify the number of protons in varying elements.
Video: Moseley's work
a. Atomic Number (Z)- The number of protons found in nucleus
b. Mass Number- The number of protons + neutrons in an atoms
-a.m.u.- atomic mass unit =
1/12 of Carbon atom ~ 1.67 x 10-24 g
-written at upper left of chemical symbol.
c. Isotopes- elements with varying number of neutrons
-to determine # of neutrons = mass number - atomic number
Resource: Isotopes of the Elements. Allows you to search for specific isotopes
Mass Spectrometers are tools used to identify isotope concentrations within an elemental sample.
Video: How mass spectrometers work
resource: How do mass spectrometers represent various atomic masses? Here. and Here (with molecular structures)
-Radioisotopes are isotopes of elements which are radioactively unstable. They undergo nuclear decay.
Uses: industry, medicine, technology, food preservation, radioactive dating and scientific research
Examples of radioisotopes used in industry, nuclear medicine
d. Atomic Mass- the average of the known masses of all isotopes and their occurrences in nature.
|
Calculating Average Atomic Mass |
|
Atomic Mass = S (Ai mi)n Ai = isotopes abundance mi = isotopes mass n = number of isotopes to averaged. |
Practice: Calculating Atomic Mass.
Practice: Finding Subatomic Particles in atoms/isotopes
Additional Links