Substitution reactions
|
Objectives: 10.2j- Write the equation for the substitution reactions of halogenoalkanes with aqueous sodium hydroxide. 20.1b- Outline the mechanism of the nucleophilic substitution reactions of halogenoalkanes with aqueous sodium hydroxide in terms of SN1 and SN2 mechanisms. 20.1c- Explain why hydroxide is a better nucleophile than water when outlining nucleophilic substitution reactions. 20.1d- Explain of how the rate of a nucleophilic reaction depends on the identity of the halogen (i.e. the leaving group), whether the halogenoalkane is primary, secondary or tertiary and the choice of solvent. 20.1e- Describe of the difference between protic and aprotic solvents. 20.1h- Write the equation for the conversion of nitrobenzene to phenylamine via a two-stage reaction. |
Introduction
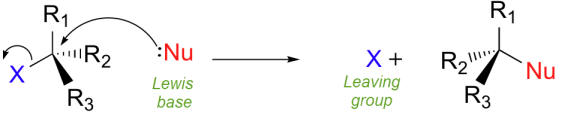
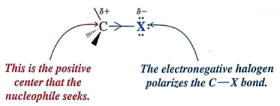
Nucleophilic substitution reactions occur when a nucleophile (Lewis base) attacks a polar carbon and causes a more electronegative group to leave (Leaving group)

Nucleophiles are structures that contain a pair of unshared electrons which are attracted to positive centers.
Resource: Three classes of nucleophiles
Resource: Nucleophile/Base strength
The leaving group should be considered a weaker base than the nucleophile, thus the reason for the substitution. These need to be stable in solution.
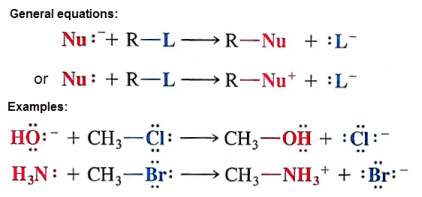
-Both Cl- and Br- are conjugate bases of strong acids, therefore are negligible bases (makes them stable).
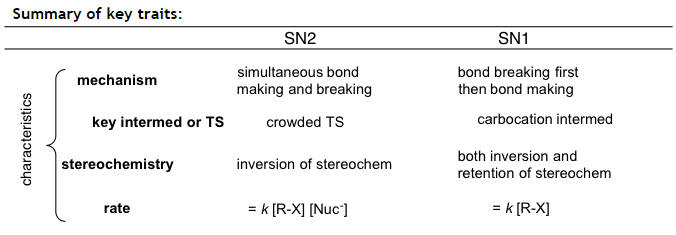
Mechanisms: SN1 vs SN2
SN2 (Hughes-Ingold mechanism)
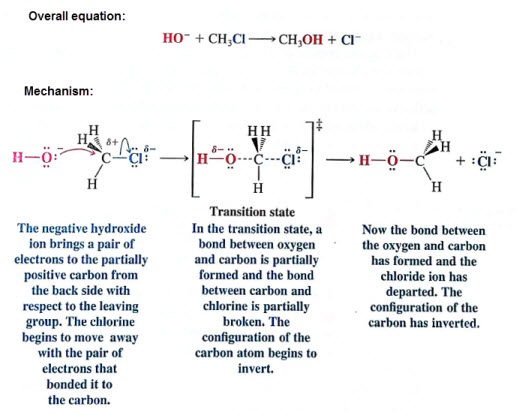
this mechanism occurs in one step- no intermediates are formed
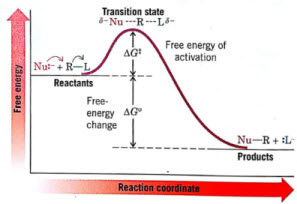
an unstable transition state forms as the nucleophile begins to form a coordinate covalent bond with the carbon and the leaving group undergoes heterolysis
this is shows second order (2o) reaction kinetics because both the substrate and nucleophilic concentrations affect the overal rate (1o for both)
Rate = k [substrate][Nu:]
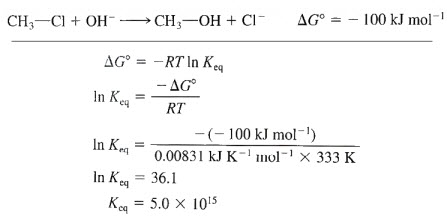
The reaction is largely exergonic (-DGo) and commonly have large Keq, which favors product formation
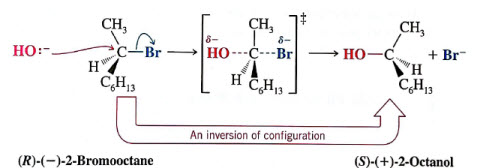
SN2 mechanisms cause a change in conformation- due to nucleophilic attack from the backside of the leaving group
SN1
These have rate equations that are unimolecular--dependent only on the concentration of the substrate (halogenalkane)
Rate = k [substrate] --> notice the [Nu:] is not part of the rate law equation
This mechanism involves a multistep process where an elementary step limits the product formation.
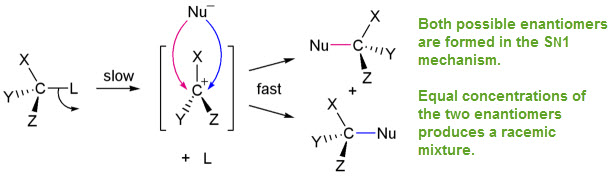
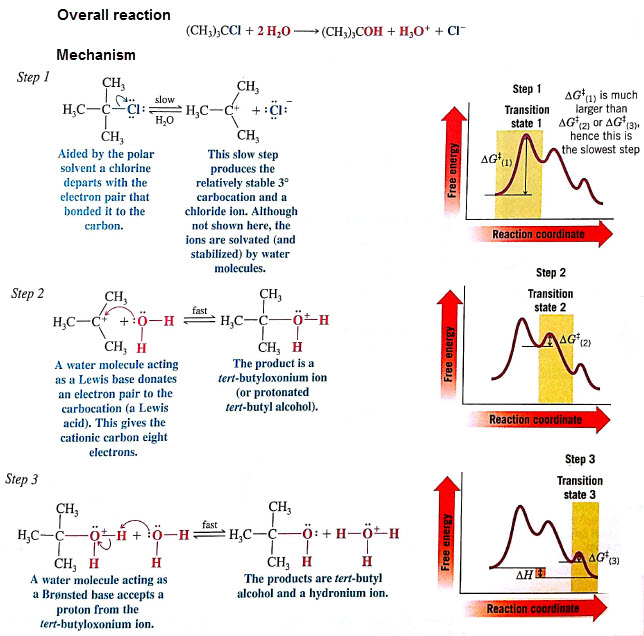
The first step is the slow step because the leaving group must leave without assistance of the nucleophile binding (SN2 )
The intermediate is a carbocation which has sp2 hybridization, leaving an empty, unhybridized p-orbital. This allows for racemic mixtures.
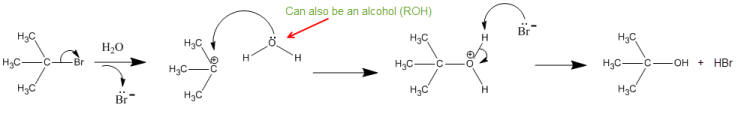
These reactions are susceptible to solvent attack, especially if the solvent is more polar.
Solvolysis- condition where the solvent can act as the nucleophile- H2O (hydrolysis), CH3OH (methanolysis)
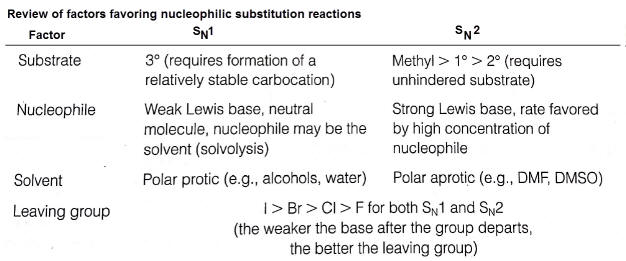
Factors that affect SN1 vs SN2 mechanisms
Two separate mechanisms exist for nucleophilic substitution reactions. These are dependent upon the following
structure of the substrate (halogenalkane)-- identify the degree of substitution (1o, 2o or 3o)
The strength of the nucleophile
the stability of the leaving group
the type of solvent
1. Structure of the substrate
SN2 - less substituted halogenalkanes proceed more readily than more substituted.
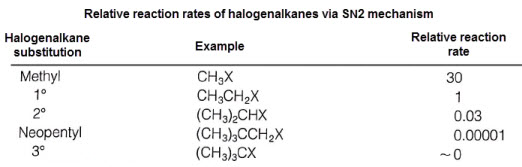
More substituted halogenalkanes exhibit greater steric hindrances- restriction of nucleophile attack on halogen-bearing carbon
The kinetic molecular theory of reactions- activation energy is dependent upon the minimum kinetic energy needed to intiate a reaction.
Steric hindrance decreasees the number of collisions with correct orientations--seen as an increase in activation energy and decrease in rate.
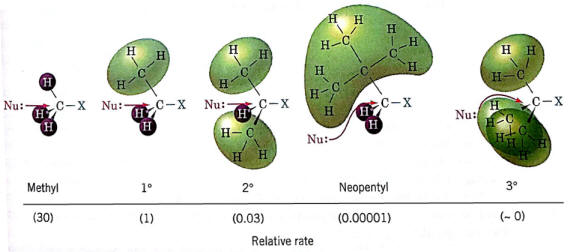
SN1- The carbocation stability is the principle factor that affects rates
only substrates that form stable carbocations undergo SN1 mechanisms--commonly 3o halogenalkanes
allylic halides (allyl group) and benzylic halides are also capable of SN1 mechanisms
3o carbocations are stabilized by hyperconjugation and the allylic/benzylic halides are stabilized through pi-bond conjugation.
2. Nucleophile strength
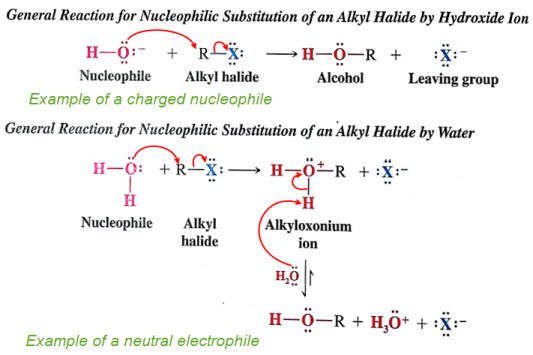
Only the SN2 mechanism rates are affected by the strength of the nucleophile.
remember for SN2 rate equations: Rate = k [substrate][Nu:], whereas for rate equations: Rate = k[substrate]
Nucleophile strength is dependent upon the following:
negatively charged nucleophiles are always stronger than its conjugate acid.
OH- is stronger than H2O and RO- is greater than ROH (RO- is an alkoxide)
the basicity of a nucleophilic atom parallels the nucleophile strength
RO- > OH- >> RCOO- > ROH > H2O
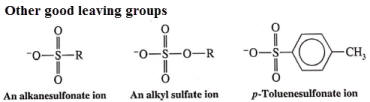
3. Leaving group
The best leaving groups form stable anions or neutral molecules--typically weak bases (conjugates of strong acids)
TsO- & NH3 >I- & H2O > Br- > Cl- >> F- > OH- & NH2- (TsO- is the tosylate ion)
These acquire and hold onto negative charge (weak bases), which stabilizes the intermediate, decreases activation energy and increases rates
trifluoromethanesulfonate ion (CF3SO3-) is one of the best leaving groups
SN1 reactions speed up with better leaving groups-
4. Solvent effects
protic solvents are defined where a hydrogen is bonded to a highly electronegative element (such as oxygen)
readily form hydrogen bonds with nucleophiles, which can inhibit both SN reactions
The strength of the hydrogen bonding is dependent upon the size of the nucleophile
smaller nucleophiles are solvated to a greater extent (form stronger attractions) and therefore reduce nucleophilic character
ex. fluoride is smaller, solvated to a greater extent and therefore is a weaker nucleophile as compared to bromide or iodide
sulfur atoms are stronger nucleophiles than oxygen species-- thiols are stronger than alcohols and sulfides are stronger than alkoxides
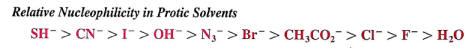
polar aprotic solvents (lack hydrogen bonded to highly e.n. atom) are used in SN2 mechanims
They dissolve ionic salts, specifically by strongly attracting to cations but not the anions, due to the lack of hydrogen bonds
the anions are free to function as strong nucleophiles
by using aprotic polar solvents, nucleophilicity of halides change-- F- > Cl- > Br- > I-
The rates of reactions greater increase in the presence of polar aprotic solvents-- up to 106 x's.

Functional group transformation
SN2 reactions provide a framework for reactions which allow for converting from one functional group to another.
This forms the basis for retrosynthetic analysis
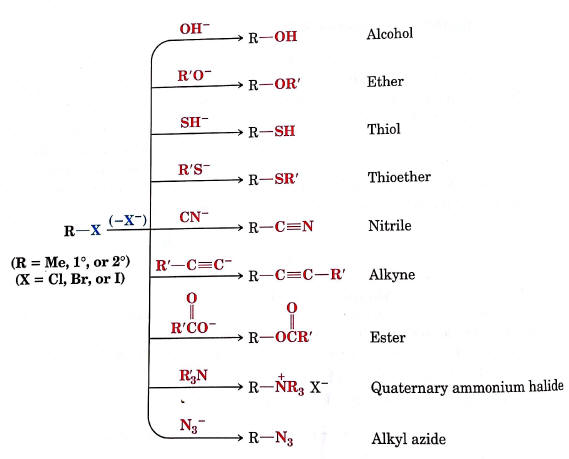
Resource: Notes on Functional group interconversions
Image: Reaction map for functional group interconversion (from LearnChemistry)
Resource: Substitution vs Elimination reactions
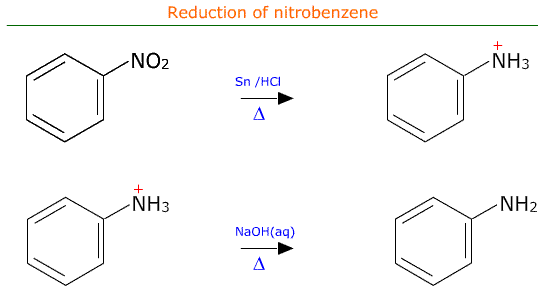
20.1h- Write the equation for the conversion of nitrobenzene to phenylamine via a two-stage reaction.