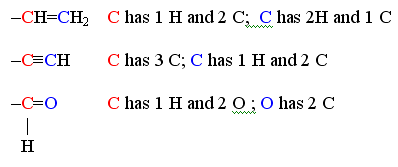Advanced
Chemistry/ IB Chemistry II- Alkanes and Cycloalkanes
IB Curriculum: Topics 10.1, 20.1,
& 20.6
I.
Defining Organic Molecules.
Organic
Chemistry- The study of
carbon-containing molecules. Although CO2 contains
carbon, it isn't considered organic but
is assimilated
into the millions of naturally occurring organic compounds via the
carbon
cycle.
Stanley Miller and
Harold Urey demonstrated that the simplest reduced form of carbon (methane: CH4)
can also be assimilated into
simple organic compounds needed to sustain life. See
Miller-Urey
Experiment.
EXOBIOLOGY: An
Interview with Stanley L. Miller
A. Organic molecules are
characterized by:
1.
Containing
tetravalent carbon atoms: always forming 4 bonds. Due to sp3
hybridization. This was proposed by
Friedrich
Kekule
- single covalent bonds possess rotational characteristics
- in organic molecules, elements possess a well-defined valency (number of
covalent bonds)
> carbon is tetravalent, oxygen is divalent, hydrogen, fluorine and chlorine are
commonly monovalent.
2.
All bonds
between atoms are covalent bonds
-some intermolecular attractions occur but are less
common due to nonpolar bonding between C-H
Read about the
Chemistry
of Carbon Bonds.
Carbon has the ability to form long chains of carbon atoms through a
process
called
catenation.
3.
Polar
bonds occur when carbon is bonded to (N, O, F, & Cl)- due to the high
differences in electronegativities
-other polar bonds are found with (H-O & H-N)
4.
Carbon
can form multiple bonds by sharing more than 1 pair of electrons with other
atoms
-based on the hybridization of carbon: double bond =
sp2 and triple bond = sp
-due to pi bonding, sp2 and sp bonded carbons are NOT free to rotate.
5.
Organic
molecules have specific three dimensional shapes
-due to # of electron dense regions- (bonded &
lone pairs of electrons about central carbons)
-VSEPR-
valence shell electron pair repulsion theory.
6.
Organic
molecules commonly contain H, N, & O (also Cl, P, & S)
Organic chemist's periodic
table
II.
Structures of Organic Molecules
Organic molecules are hydrocarbons that function as the skeletal framework of
the molecule
-hydrocarbon-
compound containing carbon & hydrogen
Molecules
differ by:
a.
structure
of the parental hydrocarbon chain (skeletal framework)
b.
addition
of functional groups: R represents any alkyl group (alkane group attached to
another)
c. geometric orientation of covalently bonded atoms
|
Formulas |
|
Formula Type |
Demonstrates |
Example: Butane |
| Empirical formula |
shows lowest ratio of elements |
C2H5 |
|
Molecular formula |
shows actual ratio of elements |
C4H10 |
|
Structural formula |
geometrically shows bonds and atoms |
|
|
Condensed structural formula |
shows functionality of each carbon |
CH3-CH2-CH2-CH3 |
>>
Fischer projection models- a 2-D visual representation of a 3-D geometric
structure. They are commonly used in biochemistry to view monosaccharides.
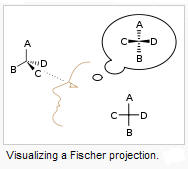
A. Isomerization
Isomers- Compounds with the same
molecular formula but having different structural formulas (molecular
structures)
-
Homologous series- a group of organic compounds that possess the same
general formula and functionality. Differences in properties are due to
increases in mass
due to the additional of a constant unit
(e.g. -CH2-)
1. structural
(constitutional)
isomers- isomers that differ by the order of attachment of their atoms
(connectivity) --
-a large variance in chemical and physical properties occur within the
homologous series (a group of isomers with common formulae)
a. skeletal (chain) isomers- molecules that differ by the composition of the
hydrocarbon skeleton (chain of carbons)
i. straight-chained-
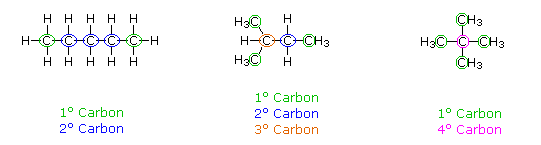
hydrocarbons where each carbon is bonded to a maximum of 2 other carbons. This
produces primary and secondary carbons.
ii. branched-chained-
hydrocarbons where a carbon atom is bonded to 3 other carbons, producing
tertiary and/or quaternary carbons.
-parent chain- the longest continual chain of carbon atoms in the molecule
|
Examples of Branched
Hydrocarbons |
|
Name |
Structural Formula |
Condensed Formula |
|
2-Methylbutane |
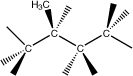
|
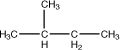
|
|
2,2-Dimethylpropane |
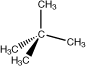
|
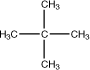
|
b. positional isomers- molecules that differ by the position of substituents on
the parent chain
-substituent- a specific atom (or group of atoms) that is/are bonded to the
parent chain
Isomers of C1
to C6 Alkanes -
c. functional isomers- molecules that differ in the composition of specific
substituents.
-functional group- a substituent that consists of a specific set of elements,
giving rise to differences in chemical and physical properties of the molecule
-moiety- another term used to identify a functional group. Multiple moieties can
be exist together to create a different functional group
i. carbon numbering- the functional group is attached to the alpha (a)
carbon in the chain.
-The next carbon in the skeleton is the beta (b),
followed by gamma (g), delta (d),
etc.
ii. functional groups by family
|
Hydrocarbon Functional Groups |
|
Family |
Functional Group |
Functional Group Structure |
Prefix/Infix/Suffix |
|
1. Alkane |
none |
none |
-ane |
|
2. Alkene |
carbon/carbon double bond |
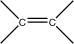
|
-ene |
|
3. Alkyne |
carbon/carbon triple bond |

|
-yne |
|
4. Arene |
resonant hexacyclic triene
(benzene ring) |
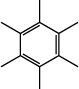
|
none |
|
5. Alkyl Halide |
carbon-halogen |

|
-none |
|
6. Alcohol |
hydroxyl |
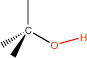
|
-ol |
|
7. Ether |
ether linkage |
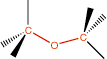
|
-oxy- |
|
8. Amine |
amine |
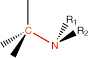 * *
|
-amine
1o, 2o & 3o |
|
9. Aldehyde |
terminal carbonyl |
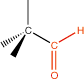
|
-al |
|
10. Ketone |
axial carbonyl |
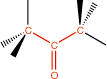
|
-one |
|
11. Carboxylic Acid |
carboxyl |
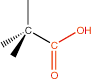
|
-oic acid |
|
12. Acid Anhydride |
dicarboxyl linkage |
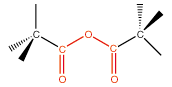 |
anhydride |
|
13. Ester |
ester linkage |
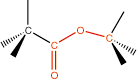
|
-ate |
|
14. Amide |
amide linkage |
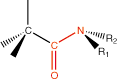 * *
|
-amide
1o, 2o & 3o
(leave e) |
|
15. Nitrile |
nitrile |
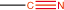
|
-nitrile |
|
16. Acyl Halide |
carbonyl w/ halide |
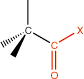
|
-ate |
|
17. Nitro compounds |
carbon-nitro |
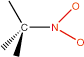
|
nitro- |
|
18. Thiols |
sulfhydryl |
-SH |
-thiol
(leave e) |
|
* R refers to any chain of carbon atoms (alkyl
group) or a single hydrogen atom. |
2. Stereoisomers- molecules with the same molecular
formula, same order of attachment (connectivity) but different geometric
structures.
a. enantiomers- molecules that are non-superposable mirror images
-superposable- objects that are able to able to coincide in space with respect
to composition and orientation
-chirality- a characteristic of an object where the mirror-image creates a non-superposable
structure
>sp3-hybridized carbons with four different substituents (within the
skeleton or not) are chiral carbons (stereocenter)
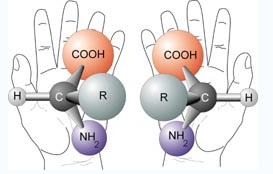
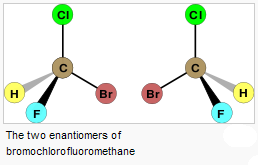
> achiral is the lack of chirality. sp3-carbons with without 4
different substituents or sp2 & sp hybridized carbons produce achiral
molecules
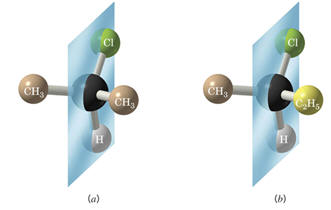
-structure (a) possesses a plane of symmetry where structure (b) has no plane of
symmetry
> a stereocenter carbon (stereogenic) possesses 4 different substituents and by
switching 2 substituents creates an enantiomeric pair
> a racemic mixtures exists when two enantiomeric pairs exist at the same
concentration.
- Polarimeters are instruments used to identify the optical activity of
substance by measuring the angle of rotation of the polarized light.
20.6.6
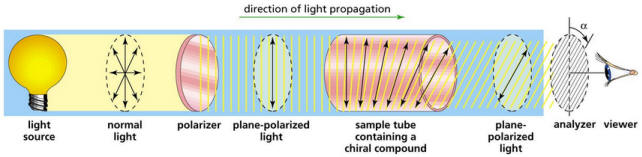
Resources:
Polarimeters --
Polarization of light
b. diastereomers- molecules that are non-superposable non-mirror images, common
in alkenes, alkynes & cyclic structures.
-These are commonly known as geometrical or "non-optical" isomers.
i. cis/trans isomers- stereoisomers that differ by the position of substituents
due to rotational restrictions
cis- Latin for "on this side"
trans- Latin for "across"

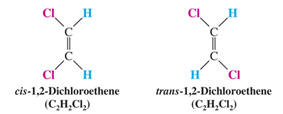
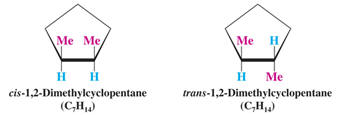
ii. multiple stereocenters- molecules that possess two or more chiral carbons
are diastereomers.
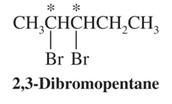
mesomers (meso compounds)- molecules that have two (or more) chiral centers that
are superposable
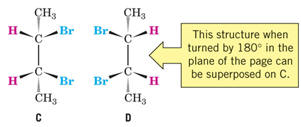
- A plane of symmetry occurs within each molecule, thus making it achiral
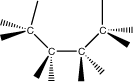
iii. conformations-
3-dimensional arrangement of atoms that result from free rotation about a single
bond (sigma bond)
-these are not really isomers because they have the same order of attachment and
geometric structure.
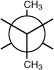
1.Staggered
conformation- rotation occurs between 2 carbon atoms until atoms on adjacent
carbons maximize their distances
Newman projection- a molecular perspective that looks
down the axis of a C -- C bond.
The second carbon is
obscured
by the first. A circle is used to represent the two carbons with 3
projections from each carbon representing the
remaining
bonds.
Visualize a
single bond rotation of butane using a Newman projection model and
comparing potential energies
2. Eclipsed
conformation- rotation that produces adjacent atoms to minimize their distances.
III.
Alkanes
Alkane- Hydrocarbon with the maximum number of hydrogen atoms per carbon (considered "saturated
with hydrogen atoms")
1.
All bonds
between carbons are single covalent bonds; sp3 hybridization
2.
The chain
of carbons must be in open form (non-cyclic structures)
-a.k.a. – saturated hydrocarbon
or aliphatic hydrocarbon. Aliphatic refers to oil or
fat-like. Properties common to larger hydrocarbons.
IV.
Naming Alkanes- Nomenclature
1. Alkane Nomenclature
IUPAC- International Union of Pure and Applied Chemistry
-
Set up
the rules by which organic compounds are named, but some common names still
exist.
Resource:
IUPAC rules (Wikipedia)
3 parts of any compound name
1. Prefix-
describes the location of substituent(s) or functional group(s)
2. Root-
describes how many carbons are in the longest chain (parent chain)
3. Suffix- identifies the functional group with the
highest precedence (family of organic compound)
For alkanes the suffix is –ane
Root nomenclature- most are named for Greek numerals except for 1,2,3 & 4
carbon molecules
|
IUPAC Prefixes of Unbranced
Alkanes |
|
# of Carbons |
Prefix |
# of Carbons |
Prefix |
# of Carbons |
Prefix |
|
1 |
meth- |
11 |
undec- |
21 |
henicos |
|
2 |
eth- |
12 |
dodec- |
22 |
docos- |
|
3 |
prop- |
13 |
tridec- |
23 |
tricos- |
|
4 |
but- |
14 |
tetradec- |
24 |
tetracos- |
|
5 |
pent- |
15 |
pentadec- |
30 |
triacont- |
|
6 |
hex- |
16 |
hexadec- |
31 |
hentriacont- |
|
7 |
hept- |
17 |
heptadec- |
32 |
dotriacont- |
|
8 |
oct- |
18 |
octadec- |
40 |
tetracont- |
|
9 |
non- |
19 |
nonadec- |
50 |
pentacont- |
|
10 |
dec- |
20 |
eicos- (icos-) |
100 |
hect- |
Alkyl Groups- alkanes attached to a parent chain- change name ending from –ane to –yl
methylene- (-CH2-) found routinely as axial methyl groups
in 3 or longer alkane chains.
The symbol R- is typically used to represent an alkyl group.
|
Names
of Common Alkyl Group |
|
Name |
Condensed Formula |
Name |
Condensed Formula |
|
methyl |
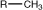
|
sec-butyl |
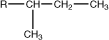
|
|
ethyl |
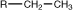
|
tert-butyl |
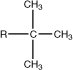
|
|
propyl |
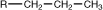
|
pentyl |
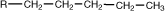
|
|
isopropyl |
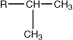
|
isopentyl |
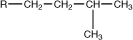
|
|
butyl |
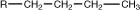
|
neopentyl |
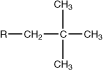
|
|
isobutyl |
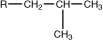
|
|
R in each structure above represents the parent chain.
This will not include any of the carbons within the alkyl group, unless
common names are used. Ex. n-butyl chloride & isopropyl alcohol
|
Glossary
of Organic Terms:
IUPAC
Nomenclature
IUPAC Rules for Naming Alkanes
1.
The
general name of an open-ended saturated hydrocarbon is an alkane
(name ending is -ane)
2.
For
branched-chain hydrocarbons, the alkane corresponding to the longest chain of
carbon atoms is taken as the
parent chain and its name is the root name
3.
Groups
attached to the parent chain are called substituents and each is given a name
and number. The number identifies
which carbon of the parent chain in which it
is attached.
-Substituents may include alkyl groups or functional groups.
4.
If there
is one substituent, number the parent chain which gives the lowest number to the
substituent carbon.
5.
If the same
substituent occurs more than once, the number of each carbon is given and the
numeric prefix for the number
groups (di-, tri-, etc.). Number the chain that yields the lowest number for
the first substituent.
6.
If there
are two or more different substituents, list them in alphabetic order and number
the parent chain to give the
lowest number for the first substituent. If two
substituents have the same position on the chain, number the chain so that
the
alphabetic first has the lower number
7.
The
prefixes di-, tri-, etc. are not used in alphabetizing, only the substituent
names.
8.
Hyphenated
prefixes, such as sec- and tert- are not considered when
alphabetizing but iso and neo, which are not hyphenated
prefixes, are considered when alphabetizing.
9.
If the hydrocarbon forms a cyclic structure, the prefix cyclo- preceeds the
prefix for the number of carbons in the cyclic structure.
|
Order of Precedence of
Functional Groups for Identifying Family Name |
|
Functional Group |
Suffix if Higher |
Prefix if Lower |
|
Carboxyl |
-oic acid |
|
|
Ester |
-R-oate |
R-oxycarbonyl- |
|
Amides |
-amide |
carbamoyl- |
|
Nitriles |
-nitrile |
cyano- |
|
Terminal Carbonyl |
-al |
oxo- |
|
Axial Carbonyl |
-one |
oxo- |
|
Hydroxyl |
-ol |
hydroxy- |
|
Thiol |
-thiol |
mercapto- |
|
Amine |
-amine |
amino- |
V. Nomenclature rules for stereoisomers 1.
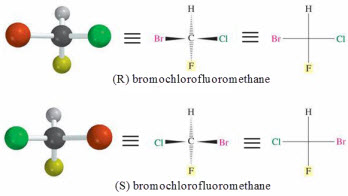
enantiomers- The configurations for enantiomer nomenclature uses the Cahn-Ingold-Prelog
convention (R,S convention)
-configurations-spatial orientations of non-equivalent groups about a chiral
carbon
a. Priority rules
i. Each atom bonded to a stereocenter is assigned a priority (higher atomic #
has higher priority)
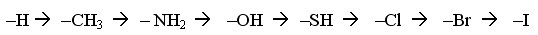
ii. If priority cannon be assigned, proceed to the next atom until a difference
exists
iii. Double and trip bonds are considered to be an equivalent number of single
bonds to the same atom
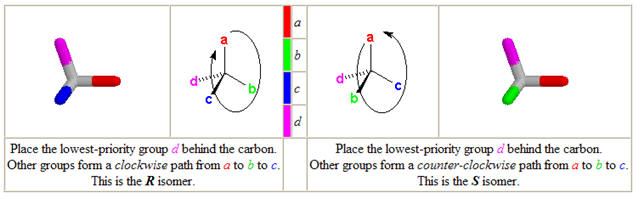
b. Assigning R, S values
1. locate the stereocenter & identify the 4 substituents
2. assign priorities to each substituent
3. Orient the structure so the lowest priority is directly behind the
stereocenter
4. Read the 3-remaining groups in order of priority
5. If priority is clockwise- R-configuration (rectus- right)
If priority is counterclockwise - S-configuration (sinister - left)
2. Naming diastereomers
a.
cis/trans-
i. skeleton orientation-
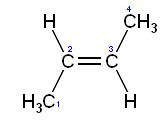
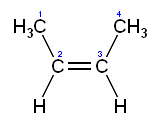
trans 2-butene
cis 2-butene
ii. substituent orientation-
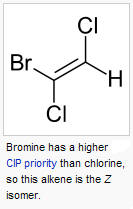
b. E/Z-
-useful when it is difficult to determine priority of substituents. Use atomic
numbers to establish priority
i. E - entgegen (German: 'opposite')
ii. Z- zusammen (German: 'together')













 *
*




 *
*