Unit 10. Acids & Bases.
Topics 8 & 18: Acids & Bases
I. Classification of substances in aqueous solutions by properties
|
A. Acids |
B. Bases |
C. Salt |
|
1. increase H3O+ concentrations 2. taste sour 3. pH < 7 4. turn blue litmus red 5. react with active metals producing H2 6. react with carbonates/bicarbonates to produce CO2 7. Neutralize bases to produce salt & water |
1. Increase OH- concentrations 2. Taste bitter 3. pH > 7 4. Turn red litmus blue 5. Have slippery feel 6. Neutralize acids to produce salt & water
|
1. ionic substance formed from the neutralization reaction of an acid and a base 2. pH ~ 7 (dependent upon strength of acid and base). See salt hydrolysis
|
A. Definitions of Acids & Bases
1. Svante Arrhenius,1883. He won the Nobel prize in 1903 for his work with electrolytic dissociation
a. Acid- Substances that increase H+ concentrations in solution
b. Base- Substances that increase OH- concentrations in solution.
2.
Bronsted-Lowry.
Developed by
Johannes
Bronsted &
Thomas Lowry.
Paper
by Bronsted (1923)
a. Acid-
Substances that donate H+ to another
b. Base- Substance that accepts H+ to another
![]()
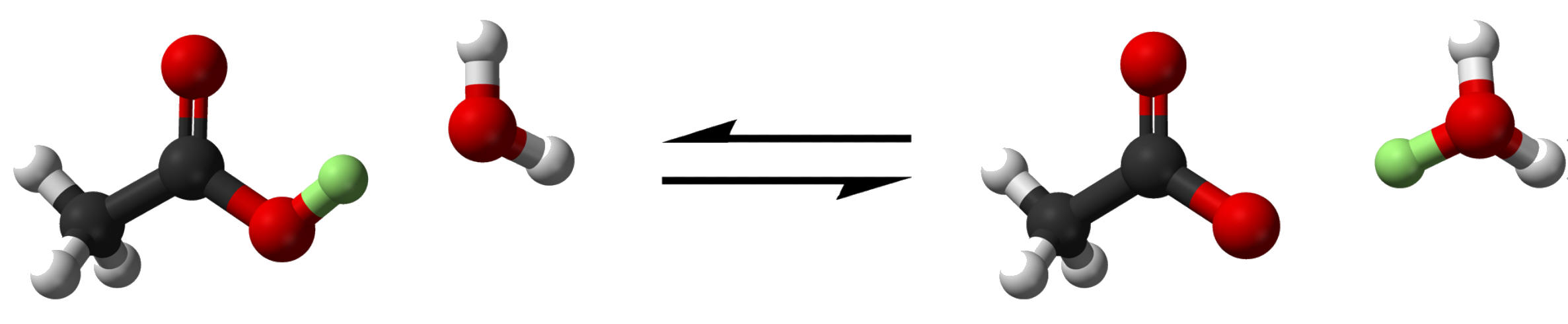
Many acid/base reactions can exist in equilibrium, so the products form acid/base pairing
c. Conjugate Acid- An acid that was created from a base
d. Conjugate Base- A base that was created from an acid
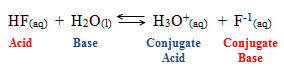
3. G. N. Lewis. Lewis Acid/Base concept
a. Acid- Substances that accept e- pairs. Often identified as an electrophile
b. Base- Substances that donate e- pairs. Often identified as a nucleophile
Animation: Acid Ionization
c. Products of Lewis acid-base chemistry are called acid/base adducts. Created through coordinate covalent bonding.
Simulation: PhET--Acids & Bases
4. Naming Acids
a. Binary acids- Acids containing a single anion bonded to a hydrogen.
ex. HCl(aq), HBr(aq), HF(aq), HI(aq), H2S(aq)
1. Naming- Use hydro- prefix and change anion name ending -ide to -ic acid
ex. HCl - hydrochloric acid
HBr- hydrobromic acid
b. Ternary acid- Acids containing polyatomic anions bonded to a hydrogen
ex. H2SO4(aq), HNO3(aq), H3PO4(aq), HC2H3O2(aq)
1. Naming- DO NOT Use hydro prefix. Change anion endings -ate to -ic acid and -ite to -ous acid.
ex. HNO3(aq) - nitric acid H2SO4(aq) -sulfuric acid
HNO2(aq) -nitrous acid H2SO3(aq) - sulfurous acid
practice: Naming acids worksheet -- answers
B. Self-ionization of water
Ionization- covalent molecules which form ions by gaining or losing protons (H+)
Self-ionization (auto-ionization) of water- Water molecules ionize to form hydroxide and hydronium ions
![]()
** At this point the term hydrogen ion, proton and hydronium will all be synonomous.
1. Ion-Product Constant for water
approximately 1 out of 550,000,000 molecules ionize
So from the Equilibrium equation:
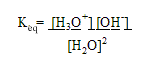 =
1.8 x 10-16
=
1.8 x 10-16
-but [H2O] ~ 55.5 M, therefore
- at 25oC, [H3O+] = [OH-]
Animation: Self-ionization of water
C.
pH & pOH scales-
measures the relative strength of an acid or base
Paper by S.P.L. Sorenson, Biochemische Zeitschrift (1909)
1. Identifying pH and pOH
pH
= -log [H3O+]
and pOH = -log [OH-]
The letter p is derived from the German word potenz meaning power or exponent of, in this case, 10
|
Concentrations and pH of various solutions |
|||
|
Solution type |
[H3O+] M |
[OH-] M |
pH Value |
|
Acidic |
> 1.0 x 10-7 |
< 1.0 x 10-7 |
< 7.00 |
|
Neutral |
= 1.0 x 10-7 |
= 1.0 x 10-7 |
= 7.00 |
|
Basic |
< 1.0 x 10-7 |
> 1.0 x 10-7 |
> 7.00 |
- In any solution, the concentrations of [H3O+] & [OH-] are inversely proportional
|
[H3O+] [OH-] = 1.0 x 10-14 -log [H3O+] [OH-] = -log ( 1.0 x 10-14) pH + pOH = 14 |
Animation: Relating pH to [H+]
Practice: Assessing pH of common materials
2. Measuring pH & Acid/Base Indicators
a. pH meters- utilize electrical potentials within a solution to measure pH.
b. Indicators: Acid-Base indicators are dyes that are themselves weak acids and bases. However, the conjugate
acid-base forms of the dye have different colors. The actual chemical structures of the dyes is often quite complex;
however, we can use the generic symbol for the indicator as HIn. The Bronsted-Lowry equation for the indicator is:
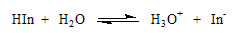
-Adding acid (increasing [H3O+]) would shift the equilibrium back to HIn
-Adding base (decreasing [H3O+]) would shift the equilibrium to In-
Image: Indicators & their color changes
Activity: Making your own pH indicator from cabbage. Anthocyanins in cabbage produces the purple color
D.
Strength of Acid or Base
Dissociation constants (Ka & Kb)- measures the amount of ionization of an acid
1. For strong electrolytes: ( like strong acids)
ex.
![]()
So 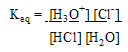 >> 1 (much larger
than one)
>> 1 (much larger
than one)
This says that in solution, the prevalent forms are H3O+ and Cl-
The [ H3O+ ] is invariably equal to [ HA ].
So if pH = -log [ H3O+ ] then for a strong acid the pH = -log [ HA ]
ex. Find the pH of a 0.015 M HCl solution
pH = -log [ H3O+ ] = -log [ HCl ] = -log (0.015) = 1.82
** The number of sig. figs. in the concentration is equal to the number of decimal places right of the decimal
practice: Finding pH of strong acids/bases -- answers
2. Weak
Electrolytes. Do they strongly
dissociate?
ex. Acetic Acid:
CH3COOH +
H2O -->
CH3COO-
+ H3O+
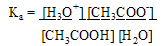
but with weak electrolytes, [H2O] remains very large and almost unchanged. IGNORE IT
Keq [H2O]
= 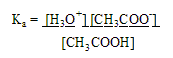 =
1.74 x 10-5 at 25oC
=
1.74 x 10-5 at 25oC
a. Calculating pH. Weak acids only partially ionize so [ H3O+ ] is not equal to [ HA ].
-To calculate the pH of weak acid or base, you must consider the Ka for the acid & Kb for the base
|
For
HA +
B -->
A- +
HB+ |
|
| When B is H2O: Keq [H2O] =
|
When HA is H2O
: Keq
[H2O] =
|
table: Strength of Acids/Bases with conjugates.
table: Ka & Kb values for common acids & bases
ex. Calculate the pH of 0.015 M Acetic Acid (CH3COOH). Construct a table to include concentrations at two
points: initial (pre-ionization, [ ]o) and final (post-ionization, [ ]i) along with changes in concentrations
| CH3COOH + H2O --> CH3COO- + H3O+ | ||||
| CH3COOH | H2O | CH3COO- | H3O+ | |
| [ ]o -initial conc. | 0.015 | - | 0 | 0 |
| D [ ] - change in conc. | - x | - | + x | + x |
| [ ]i -conc. after ionization | 0.015 - x | x | x | x |
Solution: Ka = [CH3COO-] [H3O+] ===> 1.74 x 10-5 = (x * x)
[CH3COOH] (0.015 - x)
--If the Ka << 1, assume the change in initial acid concentration is not significant, so (0.015 - x) ~ 0.015
so 1.74 x 10-5 = (x * x) ===> (1.74 x 10-5) (0.015) = x2 ===> x = 5.11 x 10-4 M
0.015
pH = -log (5.11 x 10-4) = 3.29
b. 5% Rule- As long as a weak acid or base is less than 5% ionized, then the change in initial acid/base concentration can be
ignored. If the ionization is greater than 5%, then the change in initial acid/base concentration must be included.
c. Henderson-Hasselbalch Equation.
-re: Ka is an equilibrium expression which relates the concentrations of unionized acid with concentrations of the conjugate
base and hydronium. This expression can be modified to calculate the pH of any acid/base solution knowing the
concentrations of acid and conjugate base.
|
Henderson-Hasselbalch Equation |
|
|
practice: pH including weak acids worksheet -- answers
3. Factors that affect the strength of an acid. Resource
a. Polarity and strength of H-A bond.
A more polar and/or weaker H-A bond creates greater acidic properties.
Greater polarity creates larger positive charge on the hydrogen and thus more likely the H+ is willing to be pulled away by bases/electron donors.
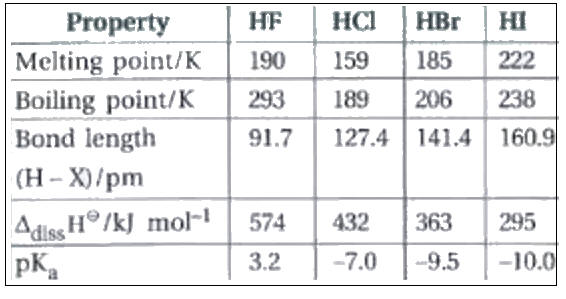
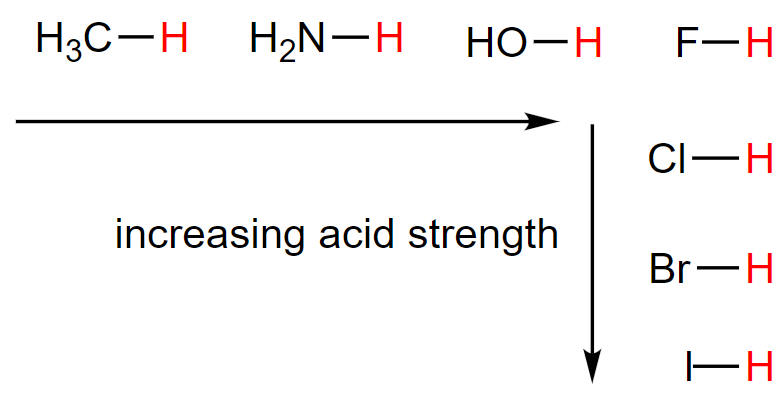
b. Conjugate base stability
An anion that is stable in water (doesn't pull H+ back due to weak or highly polar H-A bond) is a neglible base, therefore has a strong conjugate acid.
Low charge density can increase the stability of a conjugate base, commonly due to size of the anion or resonance of charge within a polyatomic anion.
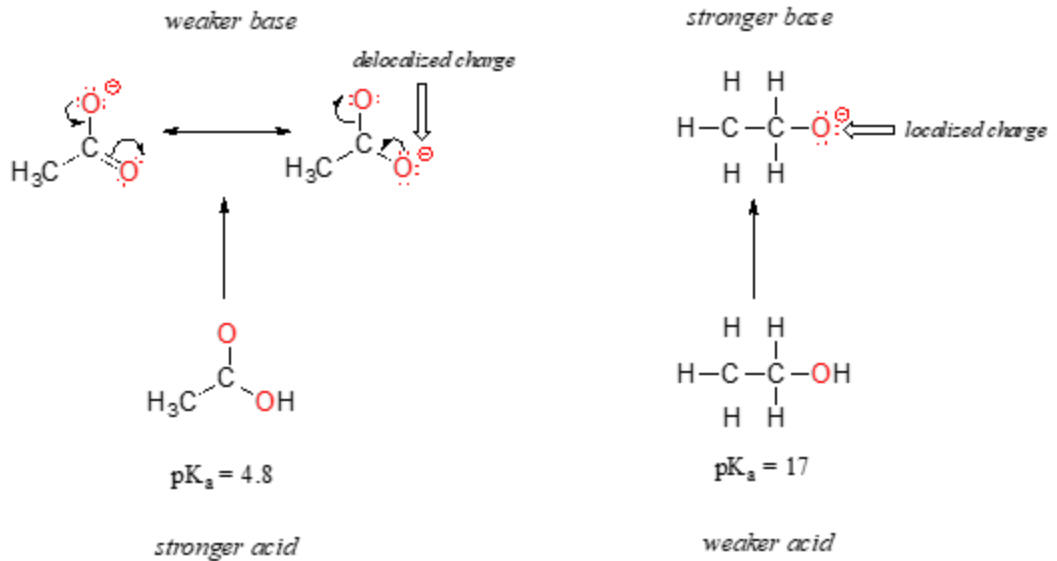
c. For oxyacids, a central atom that has a larger electronegativity or a higher oxidation state creates a stronger acid. See Table of Acid/Base strengths
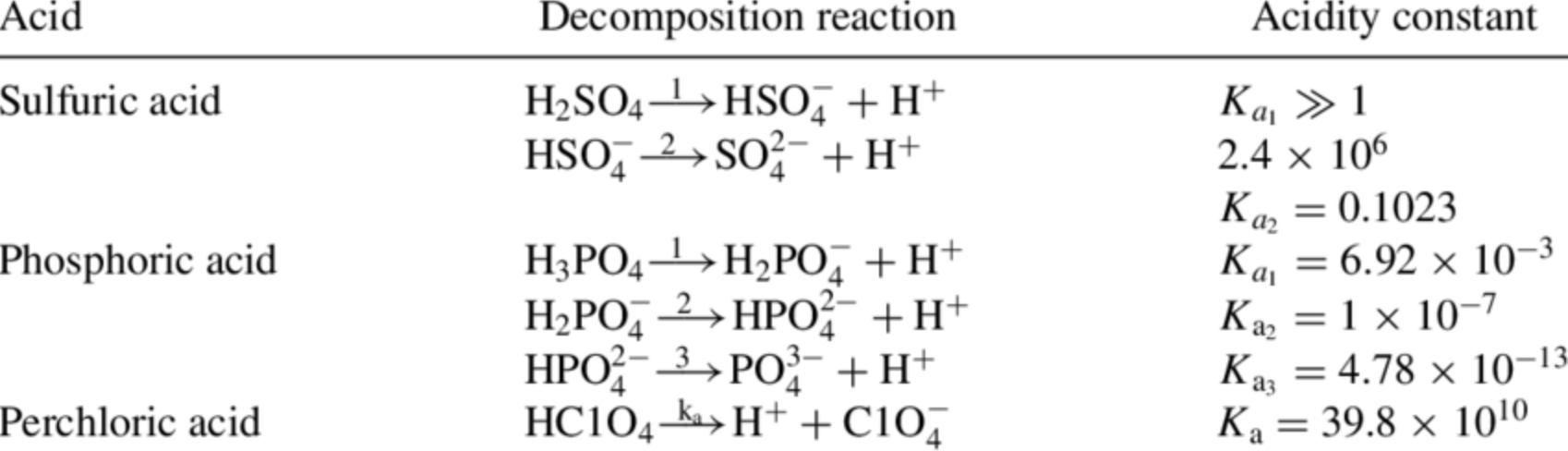

d. For carboxylic acids, peripheral atoms with high electronegativity can affect the -COO-H bond strength. This is called inductive effect.
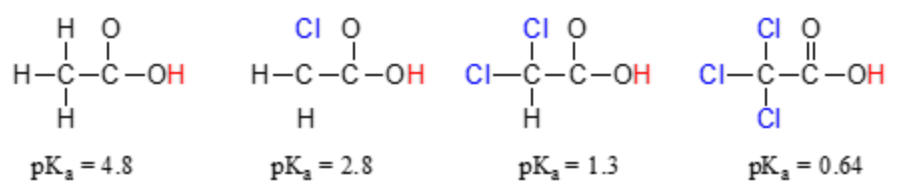
E. Acid/base neutralization
1. Acid/base neutralization reactions are double replacement reactions (metathesis) that are driven by the production of water. A soluble salt is the
other common product.
Ex. HCl(aq) + NaOH(aq) --> H2O(l) + NaCl(aq)
acid base water salt
Note: An acid neutralizes a base and vice versa but the result isn't necessarily a neutral solution. The strength of the acid/base can affect the equilibrium
and consequently affect the pH of the solution.
2. Acid-Base properties of salts
The identify of the cation (conjugate of base) and anion (conjugate of acid) can determine the acidic/basic properties of a salt solution.
a. Neutral salts are formed from cations and anions that don't affect the equilibrium of water, thus are negligible in their acidic/basic characters.
b. Basic salts contain anions that are conjugates of weak acids, therefore as weak bases will affect the equilibrium of water by binding with H+.
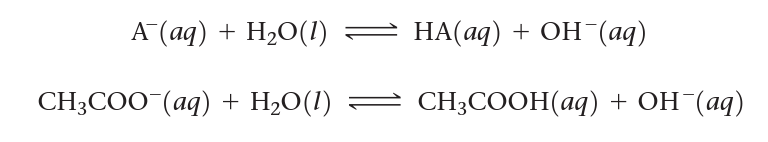
c. Acidic salts commonly contain cations that increase H3O+ in solution.
Ex. NH4Cl(aq) is an acidic salt due to the ammonium being a conjugate of a weak base.
![]()
Ex. FeCl3(aq) is an acidic salt due to the ferric ion forming a complex ion with water.
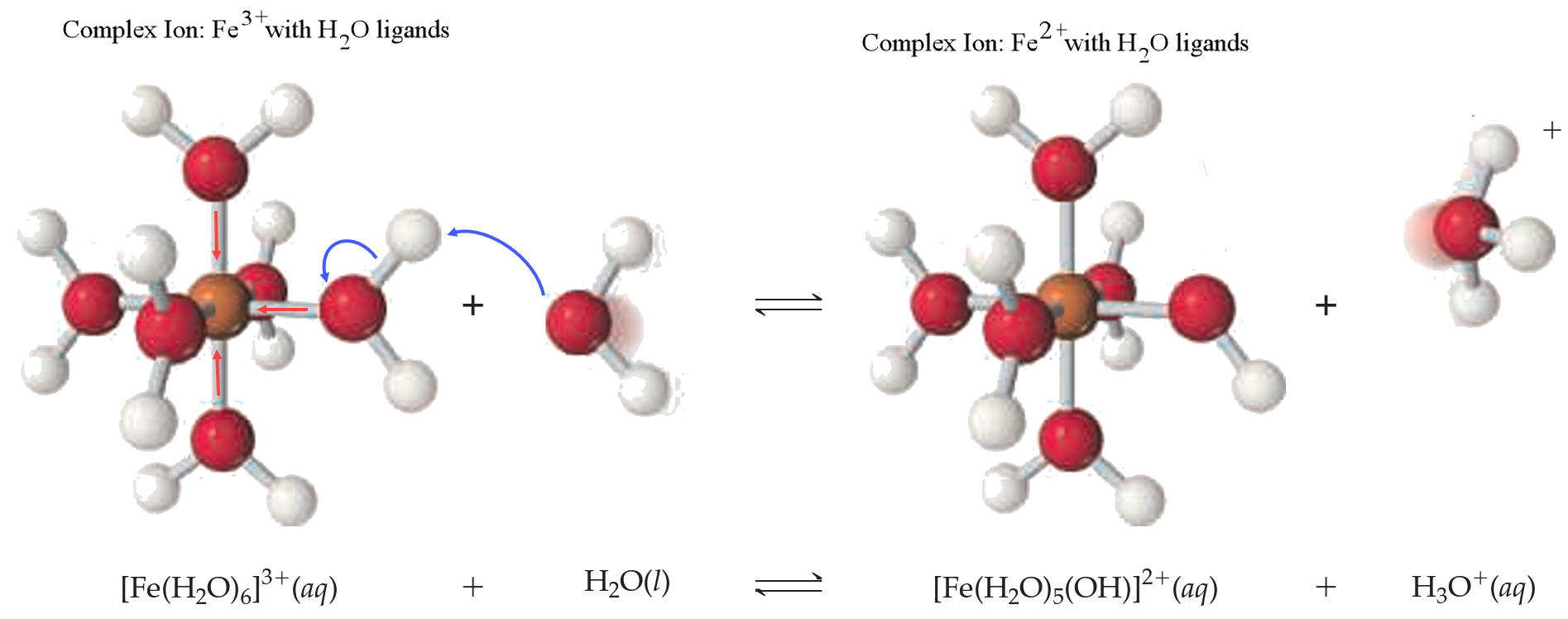
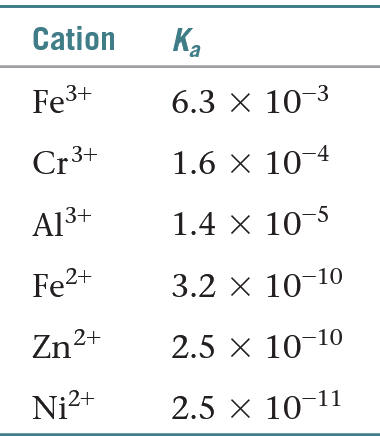
3. Titrations
An acid/base titration is a procedure used to find the concentration of an unknown acid or base. This involves three basic steps
1. Start with a specific volume of an acid or base with an unknown concentration
2. Add a acid/base indicator to the solution with the unknown concentration
3. Add the corresponding acid/base in a drop-wise fashion until a color change occurs. This represents the inflection point of a titration curve, which
is the endpoint. At this point, the moles of acid equals the moles of base.
practice: Buffers worksheet -- answers