The Properties of Water
|
Grade Level
|
|
Prospective and Practicing K-8 Teachers; may be adapted for
use in elementary classes
|
Time
 |
|
Exercises 1-4 take approximately 2 hours.
Water
 is
everywhere. It's in the air we breathe. It's in our sink faucets, and it's
in every cell of our body. Water is an unusual substance with special
properties. Just think about the wonder of water: is
everywhere. It's in the air we breathe. It's in our sink faucets, and it's
in every cell of our body. Water is an unusual substance with special
properties. Just think about the wonder of water:
|
To Ponder
 |
|
1. |
How does water rise from the roots of a redwood tree to the very top? |
| |
2. |
How do insects walk on water? |
| |
3. |
Why does ice float rather than sink?
float rather than sink? |
| |
4. |
Why do people become seriously ill, or die, if they go without
liquid
 for
a week or so? for
a week or so? |
| |
5. |
How would life in a lake be affected if ice sank and lakes froze from
the bottom up?
|
| |
In this first lab, we will investigate the properties of
water in an attempt to understand how water behaves in relation to both our
bodies and the environment. Through a concise set of experiments, the unique
properties of water and its consequent importance to living things will
become apparent.
|

|
Supplies
 |
|
chromatography paper strips
detergent
vis-a-vis black ink pens
wax paper
pennies
glue
cooking oil
red food coloring |
|
water
10 ml grad cylinders
50 ml grad. cylinders
beaker
glass slides
stirring rods
medicine droppers
scissors
|
Figure 1. Supplies

|

|
Objectives
 |
|
Once you have completed this exercise you should be able to: |
| |
1. |
Describe the
polarity
 of
a water molecule and explain how that
polarity affects the properties of water. of
a water molecule and explain how that
polarity affects the properties of water. |
| |
2. |
Explain why water climbs the inside of a thin glass capillary but not a
thin plastic capillary. |
| |
3. |
Explain why water climbs a paper strip. |
| |
4. |
Describe a system whereby the components of a water-based substance
might be separated and discuss how this separation occurs. |
| |
5. |
Explain why oil and water don't mix. |
| |
6. |
Predict whether a substance, based on its
hydrophilic
 and/or
hydrophobic properties, will dissolve into water or oil. and/or
hydrophobic properties, will dissolve into water or oil.
|

|
Background
Information
 |
|
Water covers about three fourths of the surface of the
earth? It is ubiquitous. It is also one of the simplest yet most important
molecules in living systems. It makes up from 50 to 95 percent of the weight
of living organisms. The cytoplasm of a cell is a water-based solution that
contains a variety of ions, salts, and molecules which make life 'happen.'
Water is literally involved in every facet of life.
Figure 2. Polarity of Water
Molecule

The simplicity of the water molecule belies the complexity of its
properties. Based on its small size and light weight, one can predict how it
should behave, yet it remains
liquid at a much higher temperatures than expected. It also
boils and
freezes
and
freezes at much too high, or low, of a temperature for a molecule of its size. Many
of these unexpected properties of water are due to the fact that water
molecules are attracted to each other like small magnets (cohesion)
at much too high, or low, of a temperature for a molecule of its size. Many
of these unexpected properties of water are due to the fact that water
molecules are attracted to each other like small magnets (cohesion) .
This attraction results in turn from the structure of the water molecule and
the characteristics of the atoms it contains. .
This attraction results in turn from the structure of the water molecule and
the characteristics of the atoms it contains.
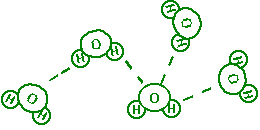
Each molecule of water is made up of two atoms of hydrogen connected to
one atom of oxygen, as shown below. This is summarized in the familiar
formula, H2O.
Figure 3. Hydrogen Bonding in
Water
|

|
Powerful Idea
 |
|
Atoms are most stable when they have a particular
configuration of their outer shells, a concept which will be discussed in
future labs. These configurations explain why hydrogen in water will take on
a partial positive charge and why oxygen will take on a
partial negative charge. These partial charges cause water
molecules to 'stick' to each other like magnets. The 'stickiness' in
this particular case is due to 'hydrogen
bonding' .
In this case, hydrogen bonding involves the attraction between the
positively charged hydrogen atom of one water molecule and the negatively
charged oxygen atom of another water molecule. As no electrons are
actually shared however, hydrogen bonds are much weaker
than covalent bonds - they easily break and easily form again. .
In this case, hydrogen bonding involves the attraction between the
positively charged hydrogen atom of one water molecule and the negatively
charged oxygen atom of another water molecule. As no electrons are
actually shared however, hydrogen bonds are much weaker
than covalent bonds - they easily break and easily form again.
|

|
Exercise 1
|
|
Surface Tension & Adhesion
|
| |
|
1a |
Drop Behavior - Water on Penny |
| To Do |
|
1. |
Obtain a medicine dropper and a small (10 ml) graduated cylinder. Make
sure the dropper is clean. |
| |
|
2. |
Drop water into the graduated cylinder with the dropper, counting each
drop. |
| |
|
3. |
How many drops, of the size produced by your medicine dropper, are in
each cubic centimeter (cc) of water? (1 cubic centimeter = 1 milliliter)?
__________ drops |
Data
Collection |
|
4. |
Conversely, how much water is in each drop? (divide 1cc by the number of
drops) __________ cc. per drop, average. |
| |
|
5. |
Now, let's see how many drops of water you can you place on the surface
of a penny before it overflows. |
Data
Collection |
|
6. |
How many drops do you predict?
Table 1. Number of Drops Predicted
| person |
#1 |
|
| person |
#2 |
|
| person |
#3 |
|
| person |
#4 |
|
| Total |
1 - 4 |
|
| Average |
|
|
|
| To Do |
|
7. |
Drop water from the dropper onto a penny, keeping careful count of each
drop. Draw a diagram below showing the shape of the water on the penny after
one drop, when the penny is about half full, and just before it overflows.
|
Data
Collection |
|
Figure 4. Drawing of Drops

|
| Results |
|
8. |
How many drops were you able to place on the surface of the penny before
it overflowed? __________ drops |
| Interpret |
|
9. |
If the number of drops is very different from your prediction, explain
what accounts for the difference.
|
| |
|
10. |
Explain your results in terms of
cohesion
|

|
| |
|
1b |
Effects of Detergent |
| To Do |
|
1. |
With your finger, spread one small drop of
detergent on the surface of a dry penny. |
| Predict |
|
2. |
How many drops do you think this penny will hold after being smeared
with
detergent, more, less, or the same as before? Why?
|
| |
|
3. |
Specifically, how many drops do you think it will hold?
Table 2. Prediction of Number of
Drops of Water on a Penny with Detergent
|
| |
|
|
| person |
#1 |
|
| person |
#2 |
|
| person |
#3 |
|
| person |
#4 |
|
| Average |
|
|
|
| To Do |
|
4. |
Using the same dropper as before, add drops of water to the penny
surface. Keep careful count of the number of drops, and draw the water on
the penny after one drop, about half full, and just before overflowing.
|
| |
|
|
Figure 5. Drawing
of Drops on a Penny with Detergent

|
| Results |
|
5. |
How many drops were you able to place on the penny before it overflowed
this time? __________ drops |
| Question |
|
6. |
Did the
detergent make a difference? Describe the effect of the
detergent.
|
| |
|
7. |
What does the
detergent do to have this effect on water?
|
| Interpret |
|
8. |
Explain how
detergents act as cleaning agents, considering the
cohesion among water molecules and the affects of
amphipathic molecules .
.
|

|
| |
|
1c |
Drop Shape on Glass and Wax Paper |
| Question |
|
1. |
What will be the shape of a drop of water on (a) a piece of wax paper
and (b) a glass slide. Draw the shape of the drop you expect on each
surface:
|
| |
|
|
| __________ |
|
__________ |
| wax paper |
|
glass |
|
| |
|
2. |
Why did you predict as you did? What assumptions are guiding your
thinking?
|
| To Do |
|
3. |
Perform the experiment. Place several drops of water on each surface and
draw the results below.
|
| Interpret |
|
|
| __________ |
|
__________ |
| wax paper |
|
glass |
|
| |
|
4. |
Compare your predictions with your observations and explain.
|
| |
|
5. |
Can you explain the differences in drop behavior in terms of
adhesion - that is, the formation (or absence) of
hydrogen bonds between molecules of different types? Which
molecules?
- that is, the formation (or absence) of
hydrogen bonds between molecules of different types? Which
molecules?
|

|
Exercise 2
|
|
The Climbing Property of Water
|
| Background |
|
1. |
Water moves to the tops of tall trees due to
capillary action combined with root pressure and evaporation
combined with root pressure and evaporation from the stomata (openings) in the leaves. Water will also climb up paper,
and often the migrating water will carry other molecules along with it. The
distance traveled by these other molecules will vary with their mass
and charge.
from the stomata (openings) in the leaves. Water will also climb up paper,
and often the migrating water will carry other molecules along with it. The
distance traveled by these other molecules will vary with their mass
and charge. |
| Predict |
|
2. |
How fast do you think water would climb a strip of absorbent paper about
one-half inch wide?
about one inch per ____________________ (time) |
| To Do |
|
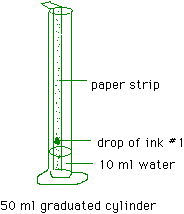
3. |
Obtain a 50 ml graduated cylinder, and tear off a strip of
chromatography paper that is just long enough to hang over the side of the
cylinder (inside) and reach to the bottom.
|
| |
|
|
Figure 6. 50 ml
Graduated Cylinder with Chromatography Paper & Ink
|
| To Do |
|
4. |
Run the paper strip along the edge of a scissors to take the curl out of
it. |
| |
|
5. |
Place a single small drop of ink from a black vis-a-vis pen on the
paper, about one inch from the bottom, and let it dry completely.
Figure 7. Ink on Chromatography
Paper

|
| |
|
6. |
Put 10 ml of water into the graduated cylinder and place the strip of
paper in the cylinder so that the bottom end is immersed in water and the
drop of ink is just above the surface of the water. Fold the paper over the
top side.
Figure 8. Close-up of Ink

|
| |
|
7. |
Note the starting time below. |
Data
Collection |
|
8. |
Watch and note the time at 5 minute intervals. When the water climbs to
the top of the paper, remove the paper from the water, and let it dry.
Table 3. Time of Water Climbing
|
| |
|
|
| Time (minutes) |
Distance (inches) |
| 0 |
|
| 5 |
|
| 10 |
|
| 15 |
|
| 20 |
|
| 25 |
|
| 30 |
|
|
| To Do |
|
9. |
How did the ink change? Glue the paper onto the page here, and label
each color on the strip.
|
| |
|
10. |
How do you explain the results? Your explanation should involve
capillary action,
polar molecules and
hydrogen bonding.
|

|
Exercise 3
|
|
Cohesion of Water
|
| |
|
3a |
Water & Oil |
| To Do |
|
1. |
Put 8 ml of water into a 10 ml graduated cylinder. |
| Predict |
|
2. |
What will happen if you add cooking oil? (Predict by choosing a, b, c,
d, or e below)
a. the oil will float on top of the water
b. the oil will sink to the bottom of the water
c. the oil will dissolve in the water
d. the oil will become mixed up with the water
e. other (what?)
|

|
| |
|
|
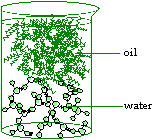
Oil is a
hydrophobic or 'water hating' molecule, so called because its
chemical structure does not allow the formation of hydrogen bonds.
Therefore, oil does not dissolve in water. When mixed, the two substances
form separate layers, and because oil is less dense, it sits on top of
water.
Figure 9. Water and Oil
|

|
| |
|
3. |
Gently add 2 ml of cooking oil by tilting the cylinder of water slightly
and letting the oil run slowly down the inside of the cylinder. |
| Results |
|
4. |
What happened?
|
| To Do |
|
5. |
Save this graduated cylinder with its contents and get a clean 10 ml
cylinder for the next experiment.
|

|
| |
|
3b |
Oil & Water |
| To Do |
|
1. |
Place 8 ml of cooking oil in a 10 ml graduated cylinder. |
| Predict |
|
2. |
What will happen when you add water? (Predict by choosing a, b, c, d, or
e below)
a. the water will float on top of the oil
b. the water will sink to the bottom of the oil
c. the water will dissolve in the oil
d. the water will become mixed up with the oil
e. other (what?)
|
| To Do |
|
3. |
Gently add 2 ml of water by tilting the cylinder of oil slightly and
letting the water run slowly down the inside of the cylinder. |
| Results |
|
|
What happened?
|
| Question |
|
4. |
Which is less dense (that is that has less weight per ml.), oil or
water? ____________________ |
| Interpret |
|
5. |
This characteristic behavior of water and oil is of critical importance
for living things, determining many properties of the cell. Can you explain
how? Consider the picture that follows:
Figure 10. Enlargement of Cell
Membrane to Show Phospholipid Bilayer.
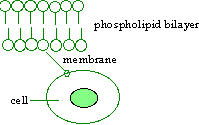
|
| Question |
|
6. |
What mechanism causes water molecules and oil molecules to separate from
one another? Your explanation should involve
polar and
non-polar molecules, the effects of
polarity on the molecular interactions, and
hydrogen bonding.
|

|
| |
|
3c |
Water, Oil, and Dye |
| Predict |
|
1. |
Predict what will happen if you add a few drops of a water-soluble dye
solution to each of the above graduated cylinders containing water and oil.
Will the dye mix with the water, the oil, or both?
|
| To Do |
|
2. |
Perform the experiment. Add a few drops of dye to each cylinder. Use a
glass stirring rod to penetrate the interface between each layer, giving the
dye access to both water and oil. How does the dye behave in each cylinder?
Does it diffuse into the oil? Into the water?
|
| Results |
|
3. |
Compare your predictions and results. Explain any differences.
|
| To Do |
|
4. |
Stir the contents of each cylinder with a stirring rod and then let it
sit. |
| Predict |
|
5. |
Will the contents remain mixed? Why do you think so?
|
| Interpret |
|
6. |
Observe what happens, compare with your prediction, and explain why it
happens. Your explanation should involve
polarity,
polar and
non-polar molecules, solution and
hydrogen bonding.
and
hydrogen bonding.
|

|
| |
|
3d |
Sheen |
| Predict |
|
1. |
Take a clean beaker of water. Predict what will happen if you add one
small drop of oil to the water using a medicine dropper. |
| To Do |
|
2. |
Do this experiment. Can you see the oil? Was your prediction correct?
Add more drops of oil if necessary to see it clearly. Describe. Your
description should focus on the separation of
polar and
non-polar layers and why that occurs.
|
| Predict |
|
3. |
Predict what will happen if you add a drop of
detergent to the beaker.
|
| To Do |
|
4. |
Now add a drop of
detergent to the beaker of water with oil on top. Record your
results |
| Interpret |
|
5. |
Compare the results with your prediction, and explain how the
detergent works in molecular terms. Your explanation should
focus on the ways in which
amphipathic molecules disrupt
cohesion.
|
| Interpret |
|
6. |
Explain some of the consequences of oil spills in the sea. What effects
do they have on sea life and bird life, and what methods are used to 'clean
up' oil spills?
|

|
Exercise 4
|
|
Class Summary
|
| To Do |
|
1. |
Summarize class results with respect to drops on a penny in the table
below.
|
| |
|
Table 4. Number of Drops on a
Penny
| Group |
# Drops without
Detergent |
# Drops with Detergent |
| 1 |
|
|
| 2 |
|
|
| 3 |
|
|
| 4 |
|
|
| 5 |
|
|
| 6 |
|
|
| 7 |
|
|
| 8 |
|
|
| Average |
|
|
|
| Interpret |
|
2. |
Explain the variation from group to group.
|
| |
|
3. |
What general conclusions can you draw from the class data?
|
| |
|
4. |
Summarize the most powerful ideas (1 to 5) you learned in this lab.
|

|
Exercise 5
|
|
Organizing Your Knowledge
|
| |
|
1. |
Describe at least one observation you have made outside the laboratory
that illustrates each phenomenon below.
|
| |
|
a. |
Polarity
|
| |
|
b. |
Hydrogen bonds
|
| |
|
c. |
Cohesion
|
| |
|
d. |
Surface tension
|
| |
|
e. |
Adhesion
|
| |
|
f. |
Capillary action
|
| |
|
g. |
Amphipathic
|
| |
|
h. |
Dissolving
|
| |
|
i. |
Density
|
| |
|
2. |
The table below summarizes nine phenomena associated with water across
the top and list the exercises we have performed down the side. For each
exercise, indicate which phenomena are illustrated.
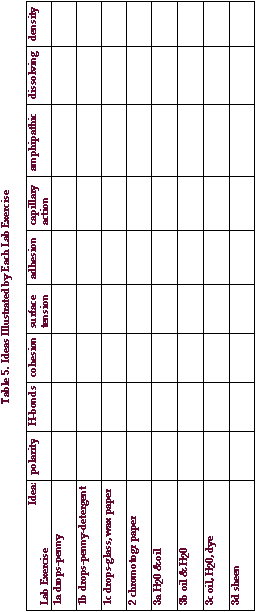
|

|
Supplementary
Resources
 |
|
The following books support the California Department of
Education's Science Frameworks.
Watson, Lyall. (1988). The Water Planet. Beautifully illustrated, this
book discusses the physics and chemistry. New York: Crown Publishers.
Dorsey, N. Ernest. (1968). Properties of ordinary water substance in all
its phases: water vapor, water & all the ices. New York: Hafner Publishing.
Wetlist
(http://www.uwin.siu.edu/WaterSites/index.html) - Comprehensive Water Topics |

|
Related
AAAS
Benchmarks
 |
|
Chapter 5: THE LIVING
ENVIRONMENT
Section C: Cells
Grade K-2 (Benchmark 2 of 2)
Most living things need water, food, and air.
Grade 3-5 (Benchmark 1 of 2)
Some living things consist of a single cell. Like familiar organisms, they
need food, water, and air; a way to dispose of waste; and an environment
they can live in.
Grade 6-8 (Benchmark 4 of 4) About two thirds of the weight of
cells is accounted for by water, which gives cells many of their properties.
Chapter 8: THE DESIGNED WORLD
Section A: Agriculture
Grade K-2 (Benchmark 1 of 4)
Most food comes from farms either directly as crops or as the animals that
eat the crops. To grow well, plants need enough warmth, light, and water.
Crops also must be protected from weeds and pests that can harm them. |
![]()
![]() is
everywhere. It's in the air we breathe. It's in our sink faucets, and it's
in every cell of our body. Water is an unusual substance with special
properties. Just think about the wonder of water:
is
everywhere. It's in the air we breathe. It's in our sink faucets, and it's
in every cell of our body. Water is an unusual substance with special
properties. Just think about the wonder of water: ![]()
![]()

![]()
![]()

![]() and
freezes
and
freezes![]() at much too high, or low, of a temperature for a molecule of its size. Many
of these unexpected properties of water are due to the fact that water
molecules are attracted to each other like small magnets (cohesion)
at much too high, or low, of a temperature for a molecule of its size. Many
of these unexpected properties of water are due to the fact that water
molecules are attracted to each other like small magnets (cohesion)![]() .
This attraction results in turn from the structure of the water molecule and
the characteristics of the atoms it contains.
.
This attraction results in turn from the structure of the water molecule and
the characteristics of the atoms it contains. 










