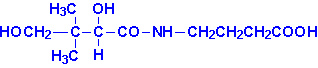Introduction to Vitamins
Vitamins are organic molecules that function in a wide variety of capacities
within the body. The most prominent function is as
cofactors for enzymatic reactions. The
distinguishing feature of the vitamins is that they generally cannot be
synthesized by mammalian cells and, therefore, must be supplied in the diet. The
vitamins are of two distinct types:
| Water Soluble Vitamins |
Fat Soluble Vitamins |
|
|
|
Return to Medical Biochemistry Page
Thiamin
 |
| Thiamin structure |
Thiamin is also known as vitamin B1
. Thiamin is derived from a substituted pyrimidine and a thiazole which
are coupled by a methylene bridge. Thiamin is rapidly converted to its active
form, thiamin pyrophosphate, TPP, in the brain
and liver by a specific enzymes, thiamin diphosphotransferase.
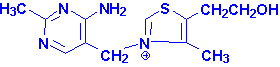
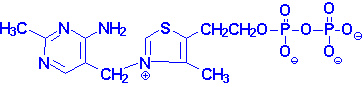 |
| Thiamin pyrophosphate |
TPP is necessary as a cofactor for the pyruvate and
a-ketoglutarate dehydrogenase catalyzed
reactions as well as the transketolase catalyzed reactions of the
pentose phosphate pathway. A deficiency in thiamin intake leads to a severely
reduced capacity of cells to generate energy as a result of its role in these
reactions.
The dietary requirement for thiamin is proportional to the caloric intake of
the diet and ranges from 1.0 - 1.5 mg/day for normal adults. If the carbohydrate
content of the diet is excessive then an in thiamin intake will be required.
back to the top
Clinical Significances of Thiamin Deficiency
The earliest symptoms of thiamin deficiency include constipation, appetite
suppression, nausea as well as mental depression, peripheral neuropathy and
fatigue. Chronic thiamin deficiency leads to more severe neurological symptoms
including ataxia, mental confusion and loss of eye coordination. Other clinical
symptoms of prolonged thiamin deficiency are related to cardiovascular and
musculature defects.
The severe thiamin deficiency disease known as Beriberi,
is the result of a diet that is carbohydrate rich and thiamin deficient. An
additional thiamin deficiency related disease is known as
Wernicke-Korsakoff syndrome. This disease is most commonly found in chronic
alcoholics due to their poor dietetic lifestyles.
back to the top
Riboflavin
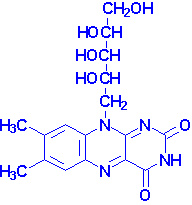 |
| Riboflavin structure |
Riboflavin is also known as vitamin B2.
Riboflavin is the precursor for the coenzymes, flavin
mononucleotide (FMN) and flavin adenine
dinucleotide (FAD). The enzymes that require FMN or FAD as cofactors
are termed flavoproteins. Several flavoproteins also contain metal ions and are
termed metalloflavoproteins. Both classes of enzymes are involved in a wide
range of redox reactions, e.g. succinate dehydrogenase and
xanthine oxidase. During the course of the enzymatic reactions involving
the flavoproteins the reduced forms of FMN and FAD are formed, FMNH2
and FADH2, respectively.
 |
Structure of FAD
nitrogens 1 & 5 carry hydrogens in
FADH2 |
The normal daily requirement for riboflavin is 1.2 - 1.7 mg/day for normal
adults.
back to the top
Clinical Significances of Flavin Deficiency
Riboflavin deficiencies are rare in the United States due to the presence of
adequate amounts of the vitamin in eggs, milk, meat and cereals. Riboflavin
deficiency is often seen in chronic alcoholics due to their poor dietetic
habits.
Symptoms associated with riboflavin deficiency include, glossitis,
seborrhea, angular stomatitis, cheilosis and photophobia. Riboflavin decomposes
when exposed to visible light. This characteristic can lead to riboflavin
deficiencies in newborns treated for hyperbilirubinemia by phototherapy.
back to the top
Niacin
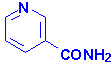 |
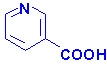 |
| Nicotinamide |
Nicotinic Acid |
Niacin (nicotinic acid and nicotinamide) is also known as
vitamin B3. Both nicotinic acid and nicotinamide can
serve as the dietary source of vitamin B3. Niacin is required for the
synthesis of the active forms of vitamin B3,
nicotinamide adenine dinucleotide (NAD+) and
nicotinamide adenine dinucleotide phosphate (NADP+).
Both NAD+ and NADP+ function as cofactors for numerous
dehydrogenase, e.g., lactate and malate dehydrogenases.
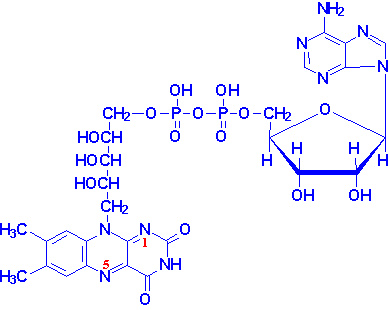
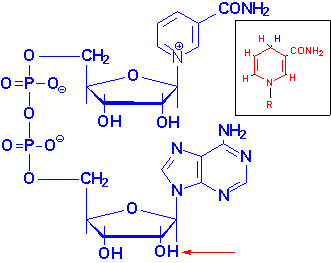 |
Structure of NAD+
NADH is shown in the box insert.
The -OH phosphorylated in NADP+ is indicated by the red arrow. |
Niacin is not a true vitamin in the strictest definition since it can be
derived from the amino acid tryptophan. However, the ability to utilize
tryptophan for niacin synthesis is inefficient (60 mg of tryptophan are required
to synthesize 1 mg of niacin). Also, synthesis of niacin from tryptophan
requires vitamins B1, B2 and B6 which would be
limiting in themselves on a marginal diet.
The recommended daily requirement for niacin is 13 - 19 niacin equivalents
(NE) per day for a normal adult. One NE is equivalent to 1 mg of free niacin).
back to the top
Clinical Significances of Niacin and Nicotinic Acid
A diet deficient in niacin (as well as tryptophan) leads to glossitis of the
tongue, dermatitis, weight loss, diarrhea, depression and dementia. The severe
symptoms, depression, dermatitis and diarrhea, are associated with the condition
known as pellagra. Several physiological
conditions (e.g.
Hartnup disease and malignant carcinoid syndrome) as well as certain
drug therapies (e.g. isoniazid) can lead to niacin deficiency. In Hartnup
disease tryptophan absorption is impaired and in malignant carcinoid syndrome
tryptophan metabolism is altered resulting in excess serotonin synthesis.
Isoniazid (the hydrazide derivative of isonicotinic acid) is the primary drug
for chemotherapy of tuberculosis.
Nicotinic acid (but not nicotinamide) when administered in pharmacological
doses of 2 - 4 g/day lowers plasma cholesterol levels and has been shown to be a
useful therapeutic for
hypercholesterolemia. The major action of nicotinic acid in this capacity is
a reduction in fatty acid mobilization from adipose tissue. Although nicotinic
acid therapy lowers blood cholesterol it also causes a depletion of glycogen
stores and fat reserves in skeletal and cardiac muscle. Additionally, there is
an elevation in blood glucose and uric acid production. For these reasons
nicotinic acid therapy is not recommended for diabetics or persons who suffer
from
gout.
back to the top
Pantothenic Acid
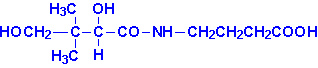 |
| Pantothenic Acid |
Pantothenic acid is also known as vitamin B5.
Pantothenic acid is formed from b-alanine and pantoic
acid. Pantothenate is required for synthesis of coenzyme A, CoA and is a
component of the acyl carrier protein (ACP) domain of fatty acid synthase.
Pantothenate is, therefore, required for the metabolism of carbohydrate via the
TCA cycle and all fats and proteins. At least 70 enzymes have been identified as
requiring CoA or ACP derivatives for their function.
Deficiency of pantothenic acid is extremely rare due to its widespread
distribution in whole grain cereals, legumes and meat. Symptoms of pantothenate
deficiency are difficult to assess since they are subtle and resemble those of
other B vitamin deficiencies.
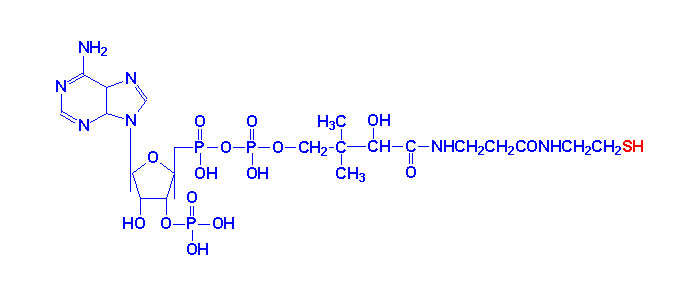 |
| Coenzyme A |
back to the top
Vitamin B6
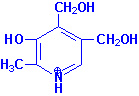 |
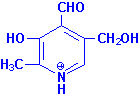 |
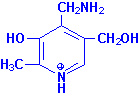 |
| Pyridoxine |
Pyridoxal |
Pyridoxamine |
Pyridoxal, pyridoxamine and
pyridoxine are collectively known as vitamin
B6. All three compounds are efficiently converted to the
biologically active form of vitamin B6,
pyridoxal phosphate. This conversion is catalyzed by the ATP
requiring enzyme, pyridoxal kinase.
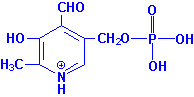 |
| Pyridoxal Phosphate |
Pyridoxal phosphate functions as a cofactor in enzymes involved in
transamination reactions required for the synthesis and catabolism of the amino
acids as well as in glycogenolysis as a cofactor for glycogen
phosphorylase. The requirement for vitamin B6 in the diet is
proportional to the level of protein consumption ranging from 1.4 - 2.0 mg/day
for a normal adult. During pregnancy and lactation the requirement for vitamin B6
increases approximately 0.6 mg/day.
Deficiencies of vitamin B6 are rare and usually are related to an
overall deficiency of all the B-complex vitamins. Isoniazid (see niacin
deficiencies above) and penicillamine (used to treat rheumatoid arthritis and
cystinurias) are two drugs that complex with pyridoxal and pyridoxal phosphate
resulting in a deficiency in this vitamin.
back to the top
Biotin
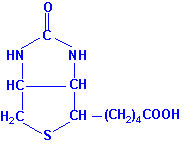 |
| Biotin |
Biotin is the cofactor required of enzymes that are involved in
carboxylation reactions, e.g. acetyl-CoA carboxylase and
pyruvate carboxylase. Biotin is found in numerous foods and also is
synthesized by intestinal bacteria and as such deficiencies of the vitamin are
rare. Deficiencies are generally seen only after long antibiotic therapies which
deplete the intestinal fauna or following excessive consumption of raw eggs. The
latter is due to the affinity of the egg white protein,
avidin, for biotin preventing intestinal absorption of the biotin.
back to the top
Cobalamin
Cobalamin is more commonly known as vitamin B12.
Vitamin B12 is composed of a complex tetrapyrrol ring structure (corrin
ring) and a cobalt ion in the center. Vitamin B12 is synthesized
exclusively by microorganisms and is found in the liver of animals bound to
protein as methycobalamin or 5'-deoxyadenosylcobalamin. The vitamin must be
hydrolyzed from protein in order to be active. Hydrolysis occurs in the stomach
by gastric acids or the intestines by trypsin digestion following consumption of
animal meat. The vitamin is then bound by intrinsic factor,
a protein secreted by parietal cells of the stomach, and carried to the ileum
where it is absorbed. Following absorption the vitamin is transported to the
liver in the blood bound to transcobalamin II.
There are only two clinically significant reactions in the body that require
vitamin B12 as a cofactor. During the catabolism of fatty acids with
an odd number of carbon atoms and the amino acids valine, isoleucine and
threonine the resultant propionyl-CoA is converted to succinyl-CoA for oxidation
in the TCA cycle. One of the enzymes in this pathway, methylmalonyl-CoA
mutase, requires vitamin B12 as a cofactor in the conversion
of methylmalonyl-CoA to succinyl-CoA. The 5'-deoxyadenosine derivative of
cobalamin is required for this reaction.
The second reaction requiring vitamin B12 catalyzes the
conversion of homocysteine to methionine and is catalyzed by methionine
synthase. This reaction results in the transfer of the methyl group from
N5-methyltetrahydrofolate to hydroxycobalamin generating
tetrahydrofolate (THF) and methylcobalamin during the process of the conversion.
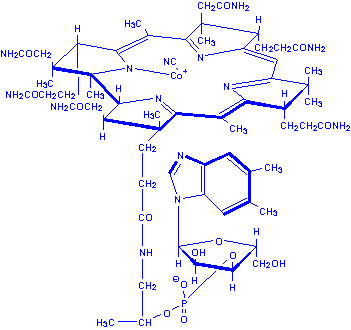 |
| Deoxyadenosylcobalamin |
back to the top
Clinical Significances of B12 Deficiency
The liver can store up to six years worth of vitamin B12, hence
deficiencies in this vitamin are rare.
Pernicious anemia is a megaloblastic anemia resulting from vitamin B12
deficiency that develops as a result a lack of intrinsic factor in the stomach
leading to malabsorption of the vitamin. The anemia results from impaired DNA
synthesis due to a block in
purine and thymidine biosynthesis. The block in nucleotide biosynthesis is a
consequence of the effect of vitamin B12 on folate metabolism. When
vitamin B12 is deficient essentially all of the folate becomes
trapped as the N5-methylTHF derivative as a result of the loss of
functional methionine synthase. This trapping prevents the
synthesis of other THF derivatives required for the purine and thymidine
nucleotide biosynthesis pathways.
Neurological complications also are associated with vitamin B12
deficiency and result from a progressive demyelination of nerve cells. The
demyelination is thought to result from the increase in methylmalonyl-CoA that
result from vitamin B12 deficiency. Methylmalonyl-CoA is a
competitive inhibitor of malonyl-CoA in fatty acid biosynthesis as well as being
able to substitute for malonyl-CoA in any fatty acid biosynthesis that may
occur. Since the myelin sheath is in continual flux the
methylmalonyl-CoA-induced inhibition of fatty acid synthesis results in the
eventual destruction of the sheath. The incorporation methylmalonyl-CoA into
fatty acid biosynthesis results in branched-chain fatty acids being produced
that may severely alter the architecture of the normal membrane structure of
nerve cells
back to the top
Folic Acid
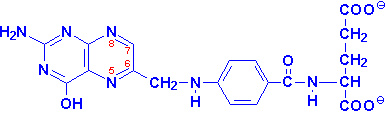 |
Folic Acid
positions 7 & 8 carry hydrogens in
dihydrofolate (DHF)
positions 5-8 carry hydrogens in tetrahydrofolate (THF) |
Folic acid is a conjugated molecule consisting of a pteridine ring structure
linked to para-aminobenzoic acid (PABA) that
forms pteroic acid. Folic acid itself is then generated through the conjugation
of glutamic acid residues to pteroic acid. Folic acid is obtained primarily from
yeasts and leafy vegetables as well as animal liver. Animal cannot synthesize
PABA nor attach glutamate residues to pteroic acid, thus, requiring folate
intake in the diet.
When stored in the liver or ingested folic acid exists in a polyglutamate
form. Intestinal mucosal cells remove some of the glutamate residues through the
action of the lysosomal enzyme, conjugase. The removal of
glutamate residues makes folate less negatively charged (from the polyglutamic
acids) and therefore more capable of passing through the basal lamenal membrane
of the epithelial cells of the intestine and into the bloodstream. Folic acid is
reduced within cells (principally the liver where it is stored) to
tetrahydrofolate (THF also H4folate) through the action of
dihydrofolate reductase (DHFR), an NADPH-requiring enzyme.
The function of THF derivatives is to carry and transfer various forms of
one carbon units during biosynthetic reactions. The one carbon units are either
methyl, methylene, methenyl, formyl or formimino groups.
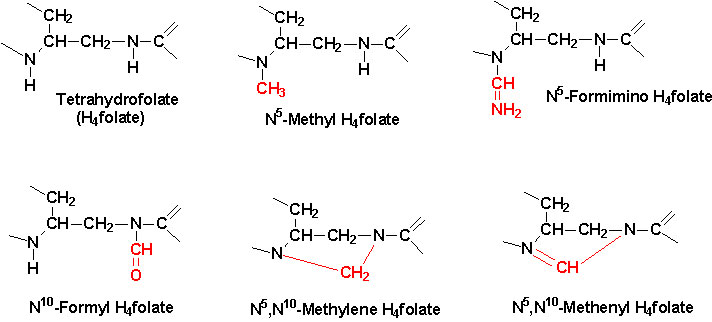 |
| Active center of tetrahydrofolate (THF).
Note that the N5 position is the site of attachment of methyl
groups, the N10 the site for attachment of formyl and formimino
groups and that both N5 and N10 bridge the methylene
and methenyl groups. |
These one carbon transfer reactions are required in the biosynthesis of
serine, methionine, glycine, choline and the purine nucleotides and dTMP.
The ability to acquire choline and amino acids from the diet and to salvage
the purine nucleotides makes the role of N5,N10-methylene-THF
in dTMP synthesis the most metabolically significant function for this vitamin.
The role of vitamin B12 and N5-methyl-THF in the
conversion of homocysteine to methionine also can have a significant impact on
the ability of cells to regenerate needed THF.
back to the top
Clinical Significance of Folate Deficiency
Folate deficiency results in complications nearly identical to those
described for vitamin B12 deficiency. The most pronounced effect of
folate deficiency on cellular processes is upon DNA synthesis. This is due to an
impairment in dTMP synthesis which leads to cell cycle arrest in S-phase of
rapidly proliferating cells, in particular hematopoietic cells. The result is
megaloblastic anemia as for vitamin B12
deficiency. The inability to synthesize DNA during erythrocyte maturation leads
to abnormally large erythrocytes termed macrocytic anemia.
Folate deficiencies are rare due to the adequate presence of folate in food.
Poor dietary habits as those of chronic alcoholics can lead to folate
deficiency. The predominant causes of folate deficiency in non-alcoholics are
impaired absorption or metabolism or an increased demand for the vitamin. The
predominant condition requiring an increase in the daily intake of folate is
pregnancy. This is due to an increased number of rapidly proliferating cells
present in the blood. The need for folate will nearly double by the third
trimester of pregnancy. Certain drugs such as anticonvulsants and oral
contraceptives can impair the absorption of folate. Anticonvulsants also
increase the rate of folate metabolism.
back to the top
Ascorbic Acid
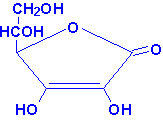 |
| Ascorbic Acid |
Ascorbic acid is more commonly known as vitamin C.
Ascorbic acid is derived from glucose via the uronic acid pathway. The enzyme
L-gulonolactone oxidase responsible for the conversion of
gulonolactone to ascorbic acid is absent in primates making ascorbic acid
required in the diet.
The active form of vitamin C is ascorbate acid itself. The main function of
ascorbate is as a reducing agent in a number of different reactions. Vitamin C
has the potential to reduce cytochromes a and c of the respiratory chain as well
as molecular oxygen. The most important reaction requiring ascorbate as a
cofactor is the hydroxylation of proline residues in collagen. Vitamin C is,
therefore, required for the maintenance of normal connective tissue as well as
for wound healing since synthesis of connective tissue is the first event in
wound tissue remodeling. Vitamin C also is necessary for bone remodeling due to
the presence of collagen in the organic matrix of bones.
Several other metabolic reactions require vitamin C as a cofactor. These
include the catabolism of tyrosine and the synthesis of epinephrine from
tyrosine and the synthesis of the bile acids. It is also believed that vitamin C
is involved in the process of steroidogenesis since the adrenal cortex contains
high levels of vitamin C which are depleted upon adrenocorticotropic hormone
(ACTH) stimulation of the gland.
Deficiency in vitamin C leads to the disease scurvy
due to the role of the vitamin in the post-translational modification of
collagens. Scurvy is characterized by easily bruised skin, muscle fatigue, soft
swollen gums, decreased wound healing and hemorrhaging, osteoporosis, and
anemia. Vitamin C is readily absorbed and so the primary cause of vitamin C
deficiency is poor diet and/or an increased requirement. The primary
physiological state leading to an increased requirement for vitamin C is severe
stress (or trauma). This is due to a rapid depletion in the adrenal stores of
the vitamin. The reason for the decrease in adrenal vitamin C levels is unclear
but may be due either to redistribution of the vitamin to areas that need it or
an overall increased utilization.
back to the top
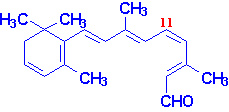
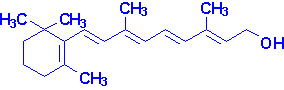
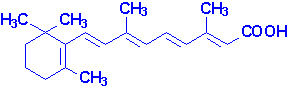
Vitamin A
Vitamin A consists of three biologically active molecules,
retinol, retinal (retinaldehyde) and retinoic
acid.
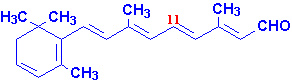 |
 |
| All-trans-retinal |
11-cis-retinal |
 |
 |
| Retinol |
Retinoic Acid |
Each of these compounds are derived from the plant precursor molecule,
b-carotene (a member
of a family of molecules known as carotenoids).
Beta-carotene, which consists of two molecules of retinal linked at their
aldehyde ends, is also referred to as the provitamin form of vitamin A.
Ingested b-carotene is cleaved in the lumen of
the intestine by b-carotene dioxygenase
to yield retinal. Retinal is reduced to retinol by retinaldehyde reductase,
an NADPH requiring enzyme within the intestines. Retinol is esterified to
palmitic acid and delivered to the blood via chylomicrons. The uptake of
chylomicron remnants by the liver results in delivery of retinol to this organ
for storage as a lipid ester within lipocytes. Transport of retinol from the
liver to extrahepatic tissues occurs by binding of hydrolyzed retinol to
aporetinol binding protein (RBP). the retinol-RBP
complex is then transported to the cell surface within the Golgi and secreted.
Within extrahepatic tissues retinol is bound to cellular
retinol binding protein (CRBP). Plasma transport of retinoic acid is
accomplished by binding to albumin.
back to the top
Gene Control Exerted by Retinol and Retinoic Acid
Within cells both retinol and retinoic acid bind to specific receptor
proteins. Following binding, the receptor-vitamin complex interacts with
specific sequences in several genes involved in growth and differentiation and
affects expression of these genes. In this capacity retinol and retinoic acid
are considered hormones of the steroid/thyroid hormone superfamily of proteins.
Vitamin D also acts in a similar capacity. Several genes whose patterns of
expression are altered by retinoic acid are involved in the earliest processes
of embryogenesis including the differentiation of the three germ layers,
organogenesis and limb development.
back to the top
Vision and the Role of Vitamin A
Photoreception in the eye is the function of two specialized cell types
located in the retina; the rod and cone cells. Both rod and cone cells contain a
photoreceptor pigment in their membranes. The photosensitive compound of most
mammalian eyes is a protein called opsin to
which is covalently coupled an aldehyde of vitamin A. The opsin of rod cells is
called scotopsin. The photoreceptor of rod cells
is specifically called rhodopsin or
visual purple. This compound is a complex
between scotopsin and the 11-cis-retinal (also called 11-cis-retinene)
form of vitamin A. Rhodopsin is a serpentine receptor imbedded in the membrane
of the rod cell. Coupling of 11-cis-retinal occurs at three of the
transmembrane domains of rhodopsin. Intracellularly, rhodopsin is coupled to a
specific G-protein called transducin.
When the rhodopsin is exposed to light it is bleached
releasing the 11-cis-retinal from opsin. Absorption of photons by 11-cis-retinal
triggers a series of conformational changes on the way to conversion
all-trans-retinal. One important
conformational intermediate is metarhodopsin II.
The release of opsin results in a conformational change in the photoreceptor.
This conformational change activates transducin, leading to an increased GTP-binding
by the a-subunit of transducin. Binding of GTP releases the
a-subunit from the inhibitory b- and
g-subunits. The GTP-activated a-subunit
in turn activates an associated phosphodiesterase; an enzyme that
hydrolyzes cyclic-GMP (cGMP) to GMP. Cyclic GMP is required to maintain the Na+
channels of the rod cell in the open conformation. The drop in cGMP
concentration results in complete closure of the Na+ channels.
Metarhodopsin II appears to be responsible for initiating the closure of the
channels. The closing of the channels leads to hyperpolarization of the rod cell
with concomitant propagation of nerve impulses to the brain.
back to the top
Additional Role of Retinol
Retinol also functions in the synthesis of certain glycoproteins and
mucopolysaccharides necessary for mucous production and normal growth
regulation. This is accomplished by phosphorylation of retinol to
retinyl phosphate which then functions similarly
to dolichol phosphate.
back top the top
Clinical Significances of Vitamin A Deficiency
Vitamin A is stored in the liver and deficiency of the vitamin occurs only
after prolonged lack of dietary intake. The earliest symptoms of vitamin A
deficiency are night blindness. Additional early
symptoms include follicular hyperkeratinosis, increased susceptibility to
infection and cancer and anemia equivalent to iron deficient anemia. Prolonged
lack of vitamin A leads to deterioration of the eye tissue through progressive
keratinization of the cornea, a condition known as xerophthalmia.
The increased risk of cancer in vitamin deficiency is thought to be the
result of a depletion in b-carotene. Beta-carotene is
a very effective antioxidant and is suspected to reduce the risk of cancers
known to be initiated by the production of free radicals. Of particular interest
is the potential benefit of increased b-carotene
intake to reduce the risk of lung cancer in smokers. However, caution needs to
be taken when increasing the intake of any of the lipid soluble vitamins. Excess
accumulation of vitamin A in the liver can lead to toxicity which manifests as
bone pain, hepatosplenomegaly, nausea and diarrhea.
back to the top
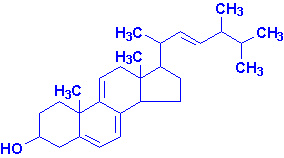
Vitamin D
Vitamin D is a steroid hormone that functions to regulate specific gene
expression following interaction with its intracellular receptor. The
biologically active form of the hormone is 1,25-dihydroxy
vitamin D3 (1,25-(OH)2D3, also
termed calcitriol). Calcitriol functions
primarily to regulate calcium and phosphorous homeostasis.
 |
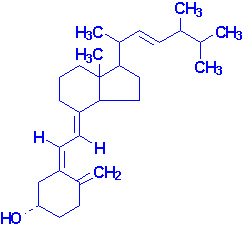 |
| Ergosterol |
Vitamin D2 |
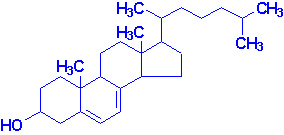 |
 |
| 7-Dehydrocholesterol |
Vitamin D3 |
Active calcitriol is derived from ergosterol
(produced in plants) and from 7-dehydrocholesterol
(produced in the skin). Ergocalciferol (vitamin
D2) is formed by uv irradiation of ergosterol. In the skin
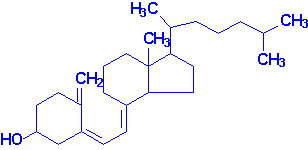
7-dehydrocholesterol is converted to cholecalciferol
(vitamin D3) following uv irradiation.
Vitamin D2 and D3 are processed to D2-calcitriol
and D3-calcitriol, respectively, by the same enzymatic pathways in
the body. Cholecalciferol (or egrocalciferol) are absorbed from the intestine
and transported to the liver bound to a specific vitamin
D-binding protein. In the liver cholecalciferol is hydroxylated at
the 25 position by a specific D3-25-hydroxylase
generating 25-hydroxy-D3 [25-(OH)D3] which is the major
circulating form of vitamin D. Conversion of 25-(OH)D3 to its
biologically active form, calcitriol, occurs through the activity of a specific
D3-1-hydroxylase present in the proximal convoluted
tubules of the kidneys, and in bone and placenta. 25-(OH)D3 can also
be hydroxylated at the 24 position by a specific D3-24-hydroxylase
in the kidneys, intestine, placenta and cartilage.
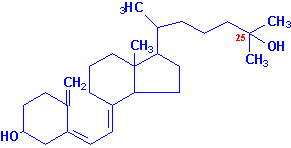 |
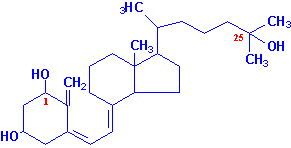 |
| 25-hydroxyvitamin D3 |
1,25-dihydroxyvitamin D3 |
Calcitriol functions in concert with parathyroid
hormone (PTH) and calcitonin to
regulate serum calcium and phosphorous levels. PTH is released in response to
low serum calcium and induces the production of calcitriol. In contrast, reduced
levels of PTH stimulate synthesis of the inactive 24,25-(OH)2D3.
In the intestinal epithelium, calcitriol functions as a steroid hormone in
inducing the expression of calbindinD28K,
a protein involved in intestinal calcium absorption. The increased absorption of
calcium ions requires concomitant absorption of a negatively charged counter ion
to maintain electrical neutrality. The predominant counter ion is Pi. When
plasma calcium levels fall the major sites of action of calcitriol and PTH are
bone where they stimulate bone resorption and the kidneys where they inhibit
calcium excretion by stimulating reabsorption by the distal tubules. The role of
calcitonin in calcium homeostasis is to decrease elevated serum calcium levels
by inhibiting bone resorption.
back to the top
Clinical Significance of Vitamin D Deficiency
As a result of the addition of vitamin D to milk, deficiencies in this
vitamin are rare in this country. The main symptom of vitamin D deficiency in
children is rickets and in adults is
osteomalacia. Rickets is characterized improper
mineralization during the development of the bones resulting in soft bones.
Osteomalacia is characterized by demineralization of previously formed bone
leading to increased softness and susceptibility to fracture.
back to the top
Vitamin E
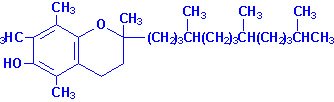 |
| a-Tocopherol |
Vitamin E is a mixture of several related compounds known as
tocopherols. The a-tocopherol
molecule is the most potent of the tocopherols. Vitamin E is absorbed from the
intestines packaged in chylomicrons. It is delivered to the tissues via
chylomicron transport and then to the liver through chylomicron remnant uptake.
The liver can export vitamin E in VLDLs. Due to its lipophilic nature, vitamin E
accumulates in cellular membranes, fat deposits and other circulating
lipoproteins. The major site of vitamin E storage is in adipose tissue.
The major function of vitamin E is to act as a natural
antioxidant by scavenging free radicals and molecular oxygen. In
particular vitamin E is important for preventing peroxidation of polyunsaturated
membrane fatty acids. The vitamins E and C are interrelated in their antioxidant
capabilities. Active a-tocopherol can be regenerated
by interaction with vitamin C following scavenge of a peroxy free radical.
Alternatively, a-tocopherol can scavenge two peroxy
free radicals and then be conjugated to glucuronate for excretion in the bile.
back to the top
Clinical significances of Vitamin E Deficiency
No major disease states have been found to be associated with vitamin E
deficiency due to adequate levels in the average American diet. The major
symptom of vitamin E deficiency in humans is an increase in red blood cell
fragility. Since vitamin E is absorbed from the intestines in chylomicrons, any
fat malabsorption diseases can lead to deficiencies in vitamin E intake.
Neurological disorders have been associated with vitamin E deficiencies
associated with fat malabsorptive disorders. Increased intake of vitamin E is
recommended in premature infants fed formulas that are low in the vitamin as
well as in persons consuming a diet high in polyunsaturated fatty acids.
Polyunsaturated fatty acids tend to form free radicals upon exposure to oxygen
and this may lead to an increased risk of certain cancers.
back to the top
Vitamin K
The K vitamins exist naturally as K1 (phylloquinone) in green
vegetables and K2 (menaquinone) produced by intestinal bacteria and K3
is synthetic menadione. When administered, vitamin K3 is alkylated to
one of the vitamin K2 forms of menaquinone.
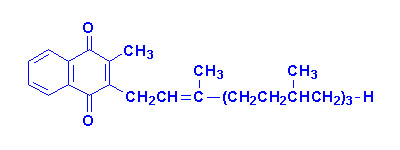 |
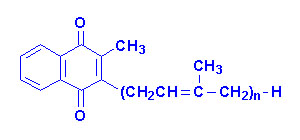 |
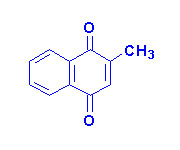 |
| Vitamin K1 |
Vitamin K2
"n" can be 6, 7 or 9 isoprenoid groups |
Vitamin K3 |
The major function of the K vitamins is in the maintenance of normal levels
of the
blood clotting proteins, factors II, VII, IX, X
and protein C and protein S,
which are synthesized in the liver as inactive precursor proteins. Conversion
from inactive to active clotting factor requires a
posttranslational modification of specific glutamate (E) residues. This
modification is a carboxylation and the enzyme responsible requires vitamin K as
a cofactor. The resultant modified E residues are
g-carboxyglutamate (gla). This
process is most clearly understood for factor II, also called
preprothrombin. Prothrombin is modified preprothrombin. The gla
residues are effective calcium ion chelators. Upon chelation of calcium,
prothrombin interacts with phospholipids in membranes and is proteolysed to
thrombin through the action of activated factor X (Xa).
During the carboxylation reaction reduced hydroquinone form of vitamin K is
converted to a 2,3-epoxide form. The regeneration of the hydroquinone form
requires an uncharacterized reductase. This latter reaction is the site of
action of the dicumarol based anticoagulants
such as warfarin.
back to the top
Clinical significance of Vitamin K Deficiency
Naturally occurring vitamin K is absorbed from the intestines only in the
presence of bile salts and other lipids through interaction with chylomicrons.
Therefore, fat malabsorptive diseases can result in vitamin K deficiency. The
synthetic vitamin K3 is water soluble and absorbed irrespective of
the presence of intestinal lipids and bile. Since the vitamin K2 form
is synthesized by intestinal bacteria, deficiency of the vitamin in adults is
rare. However, long term antibiotic treatment can lead to deficiency in adults.
The intestine of newborn infants is sterile, therefore, vitamin K deficiency in
infants is possible if lacking from the early diet. The primary symptom of a
deficiency in infants is a hemorrhagic syndrome.
back to the top