13.5 Reactions of Alkenes & Alkynes
A. Addition Reaction: The
addition of atoms/groups of atoms across the C=C.
-The pi bond is broken and replaced
by 2 sigma bonds. This requires changes the hybridization states of the central
carbons.
1. Reaction Mechanism: A description
of which bonds are broken and which bonds are formed, along with the relative
order of each step.
-can also describe the roles of
solvents & catalysts
a. Potential Energy Diagrams &
Transition states
Consider: A +
B --> C + D
the potential energy is the energy
stored in the chemical bonds due to the position of electrons in atomic and
molecular orbitals. Remember, electrons closer to a nucleus/bonded nuclei
represent lower energy. So electrons in pi bonds which are further from
the bonded nuclei, on average, represent a higher energy state.
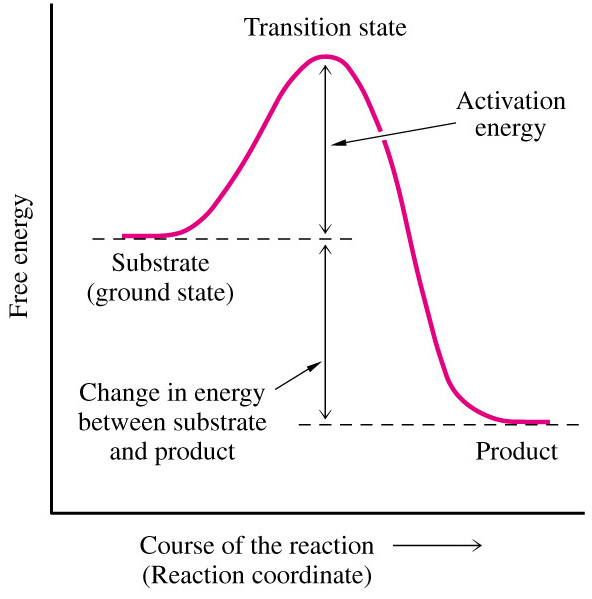 |
Activation Energy: The energy associated with transition
state formation. Attributed to reactant bond changes.
Heat of reaction (QR or DH):
The difference in potential energies between the reactants and products.
If DH
is negative (P.E.products < P.E.reactants) this is
called an exothermic reaction. If DH
is positive (P.E.reactants< P.E.products ) this is
called an endothermic reaction.
Transition State. The species within a reaction that represents a hybrid
between the reactant and product. This high-energy particles is not
stable and therefore either decomposes into product or back to reactant. The
"bonds" within a transition state complex represents strained bonds or atoms
that exceed the octet thus producing an unstable entity. |
