Review- Acid/Base theory
Brønsted-Lowry
Acid- Substance that increases [H+]
Brønsted-Lowry
Base- Substance that decreases [H+]
pH = -log[H+] ; higher [H+] = lower pH
Equilibrium
Constant Keq = [products]
[reactants]
 for self-ionization of water H2O D H+ + OH-
for self-ionization of water H2O D H+ + OH-
Keq =
[ H+ ] [OH-] for [H2O] =
1000g/L = 55.5 M
[H2O] 18 g/mol
given
that [ H+ ] = [OH-] = 1.0 x 10-7 M
then Keq = (1.0 x 10-7)(
1.0 x 10-7) = 1.8 x 10-16
55.5
therefore [
H+ ] [OH-] =(Keq )(55.5) = (1.8 x 10-16)(55.5) = 1.0 x 10-14 = Kw
Kw à Ion Product of water. This describes that the concentration of
hydrogen ions
and hydroxide ion are inversely proportional.
Can we calculate pH when [ H+ ] = [OH-]
pH
= - log [ H+ ] = -
log (1.0 x 10-7) = 7
So, when [ H+
] = [OH-] , the solution is neutral.
Ionization
Constant (Ka)- describes the ability of an acid to form ions in
solution.
For strong electrolytes: ( like strong
acids)
HCl + H20 à H3O+ + Cl-
So Keq = [ H3O+ ] [Cl- ] >> 1 (much larger than one)
[ HCl ][ H20 ]
So… this says that in solution, the
prevalent forms are H3O+ and Cl-
Weak Electrolytes. Do they strongly dissociate?
Acetic Acid (CH3COOH)
CH3COOH + H2O à CH3COO- + H3O+
Keq = [CH3COO- ][H3O+]
, but with weak electrolytes, [H2O]
remains
[CH3COOH][H2O] very large and almost unchanged. IGNORE
IT.
Ka = [CH3COO- ][H3O+]
=
1.74 x 10-5
[CH3COOH]
So what does this mean?
Relating
pH and Ka values. The
Henderson-Hasselbalch Equation
Ka describes the equilibrium concentrations of the
ionic forms and acid forms.
What does pH depend on?
The amount of H+ in
solution is dependent upon the amount of acid that has been
dissociated.
In other words [H+]
= [A-], given the equation:
HA + H2O à H3O+ +
A-
Deriving
Henderson-Hasselbalch,
Ka = [A- ][H3O+]
[HA]
[H3O+] = Ka [HA]
[A- ]
Take (-log) of both sides pH
= pKa - log [HA]
[A-]
pH
= pKa + log [A-]
[HA]
Can we find the pH when a weak acid is
½ dissociated if we know the Ka ?
pH = -log Ka when [A-]= [HA], because if [A-] = [HA], then log 1 = 0
pH of HAc (@ [Ac-] = [HAc]) = - log (1.74 x 10-5) = 4.76
Does the initial concentration of HCl
affect the pH?
Does the initial concentration of HAc
affect the pH?
Changes
in pH as a function in changes in [A-] or [HA]
What happens if the pH of an amino acid solution is decreased? Increased?
Example. If the pH is 2 units below the pKa
pH = pKa + log [A-] à pH = pKa +(-2)
[HA]
therefore: log [A-] = -2
[HA]
(take 10^ of both sides) [A-] = 0.01
[HA]
What does this mean?
The pKa of Formic Acid is
3.75. Calculate using the
Henderson-Hasselbalch equation the pH of a solution containing 200 mL of 0.1 M Formic
acid and 150 mL of 0.1 NaOH with enough water bring the entire solution volume
to 1 L.
Titrations
of Amino Acids
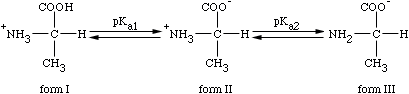
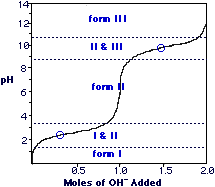
What do pKa1 & pKa2 mean?
Where does the Isoelectric point exist? What does it mean?
Solving
for Isoelectric point
pI = pKa1 + pKa2
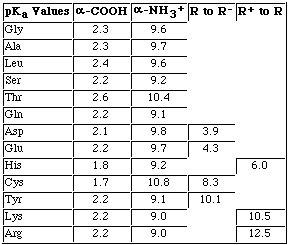
2
For alanine, the pI equals
pI = 2.3 + 9.7 = 6
2
So, what does this mean?

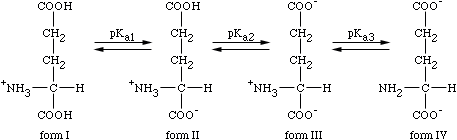
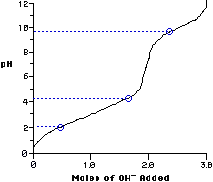
Calculate the pI for Glutamic Acid.