Advanced Chemistry: Ch 18. Amino Acids & Proteins
A.
Why Biochemistry?
Chemistry is the logic of Biological
Phenomena……… Huh?
1.
Hierarchy
of Life: Living Organisms can be
described by levels of complexity.
a.
Organism
b.
Systems-
Respiratory, Circulatory, Lymphatic, etc.
c.
Organs-
heart, lung, kidney, etc.
d.
Tissues-muscle,
connective, epithelial, nerve
e.
Cells-
prokaryotic, eukaryotic, specialized eukaryotic, RBC
f.
Organelles
& Membranes
g.
Macromolecules
1.
Supramolecular
complexes- Ribosomes, cytoskeleton, enzyme complexes
2.
Macromolecules-
proteins, nucleic acids, polysaccharides, lipids
3.
Building
blocks- amino acids, nucleotides, monosaccharides, fatty acids
4.
Metabolites-
pyruvate, citrate, succinate, phosphoglyceraldehyde
5.
Inorganic
Precursors- CO2, NH3, H2O, N2
h.
Atoms
2.
Composition
of Biomolecules (In Human body by % composition)
a.
Hydrogen
63%
b.
Oxygen
25.5%
c.
Carbon
9.5%
d.
Nitrogen
1.4%

3.
Why
do all biomolecules contain carbon? Reason for high degree of variability
a.
Ability
to form stable covalent bonds with itself- long chains
b.
Tetrahedral
arrangement creates vast number of possible molecules
4.
What
functional groups are prevalent in biomolecules?
a.
Amino
b.
Hydroxyl
c.
Carbonyl
d.
Carboxyl
e.
Amide
f.
Ester
g.
Phosphate
ester
h.
Hemiacetal
i.
Acetal
B.
Proteins: An overview
The most prevalent group of biomolecules
(50 % of dry body weight) and the most diverse
All proteins are made mostly from 20
naturally occurring Amino Acids
1.
Types
of Proteins
a.
Enzymes-
Ribonuclease, Trypsin, Alcohol Dehydrogenase
b.
Regulatory-
Insulin, lac Repressor
c.
Transport-
Hemoglobin, Serum Albumin
d.
Storage-
Ovalbumin, Casein, Ferritin
e.
Contractile
& Motile- Actin/Myosin, Tubulin/Dynein
f.
Structural-
a-Keratin,
Collagen, Elastin
g.
Scaffold-
(used during cellular response mechanisms)
h.
Protective
& Exploitive- Immunoglobulins, Thrombin/Fibrinogen, Ricin, Venoms
i.
Exotic-
Monellin, Glue Proteins
2.
How
many proteins are required for life?
a.
Mycoplasma
genitalium (causes clamdyia)- 468 proteins
b.
Haemophilus
influenza (causes bacteria meningitis)- 1703 proteins
c.
Escherichia
Coli- ~4000 proteins
d.
Yeast-
~5000 proteins
e.
Worms-
~20,000 proteins
f.
Humans-
50,000-100,000 proteins
Minimal for survival, “essential proteins”- 256
proteins
C.
Amino Acids
1.
General
Structure-
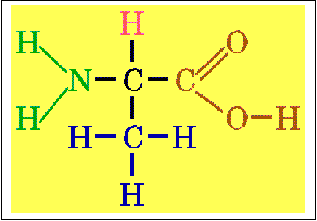
a.
![]() a-carbon (central carbon)
a-carbon (central carbon)
b.
a-Amino Group
c.
a-Carboxyl Group same for all amino acids (except Proline)
d.
Hydrogen
e.
Side
Chain (R)- Gives rise to variability in amino acids
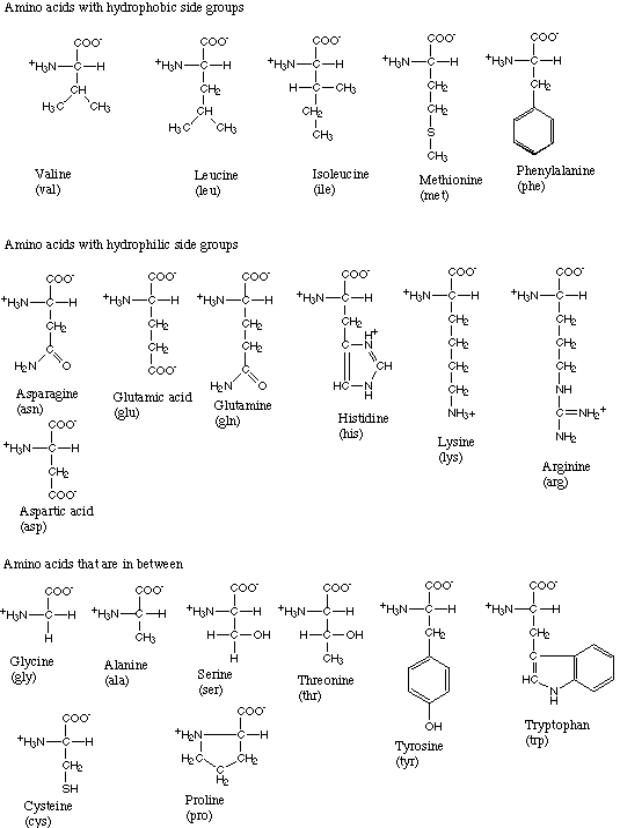
2. Common Amino Acids
a. Nonpolar or Hydrophobic
1.
Alanine
(Ala, A)
2.
Valine
(Val, V)
3.
Leucine
(Leu, L)
4.
Isoleucine
(Ile, I)
5.
Methionine
(Met, M)
6.
Proline
(Pro, P)
7.
Phenyalanine
(Phe, F)
8.
Tryptophan
(Trp, W)
b. Polar, Uncharged
1.
Glycine
(Gly, G)
2.
Serine
(Ser, S)
3.
Asparagine
(Asp, N)
4.
Glutamine
(Glu, Q)
5.
Threonine
(Thr, T)
6.
Tyrosine
(Tyr, Y)
7.
Cysteine
(Cys, C)
c. Acidic
1.
Asparatic
Acid (Asp, D)
2.
Glutamic
Acid (Glu, E)
d. Basic
1. Histidine (His, H)
2.
Lysine (Lys, K)
3.
Arginine
(Arg, R)
3. Properties
a. Acid-Base Properties
-Amino Acids are polyprotic acids
(release more than one H+) = Dissociable protons
The amino functions as a
Lewis Base & carboxyl functions as a Lewis Acid, therefore all amino acids
undergo an internal Acid-Base Reaction where the H+ is dissociated from the
carboxyl forming a carboxylate and attached through a coordinate covalent bond
to the a-nitrogen thus forming an ammonium ion.
-The dipolar molecule formed
(being electrically neutral) is called a Zwitterion
(German: “Hybrid ion”).
The Zwitterion of Life by SDGreen
Like a zwitterion,
Sometimes life can have a positive charge;
Perhaps a good score on a test,
Or another accomplishment that is large.
But also like a zwitterion,
Life has its negative sides,
This can be breaking up with someone you care,
Or being taken on pointless rides.
But like a zwitterion,
We must accept both good and bad,
Because somewhere in this structure,
Is a complete life to be had.
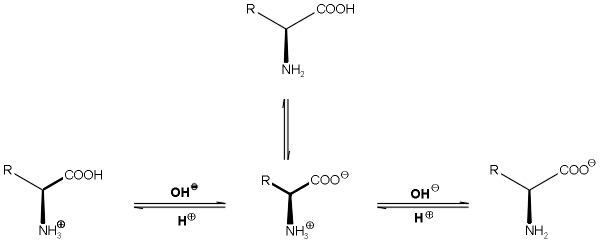
Cationic Form Zwitterion Anionic Form
b. Stereochemistry
1. D,L- system for identify
stereoisomerization
D- Dextrorotary- clockwise
rotation of plane-polarized light
L- Levorotary- counterclockwise
rotation of plane-polarized light
Almost all amino acids that
comprise proteins are in the L form.
2. R,S- system
replacing
the D,L system due to amino acids with multiple chiral carbons
All a-carbon configurations of the L-amino acids are (S) except for
cysteine, by virtue of the thiol group, and it is (R ).
3. Special Note. Fisher Projection models
These are two dimensional
drawing that represent 3-dimensional structures.
![]() COO-
COO-
Substituents
above and below are behind the plane with
![]()
![]() H3N+ C H with a-carbon
H3N+ C H with a-carbon
![]() Substituents left
and right are in front .
Substituents left
and right are in front .
CH3
COO-
![]()
![]()
![]() H3N+ C H What
enantiomeric form of alanine does this represent?
H3N+ C H What
enantiomeric form of alanine does this represent?
![]()
CH3 Draw the other enantiomeric form of alanine.
D.
Protein Structures
1. Primary Structure- Linear sequence of
amino acids (residues)
a. Peptide bond- bond between carboxyl and adjacent
amino groups. Formed through a
condensation reaction
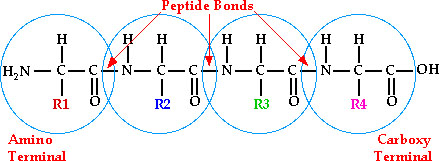
all
primary sequences are read from the N-terminus to the C-terminus.
So if R1 = methionine, R2=
threonine, R3 = proline and R4 = valine, then the sequence is named
![]()
![]()
met-thr-pro-val or can be named MTPV
Characteristics
of the peptide bond (amide linkage)
-
coplanar
-
polar
-
no
rotation (means some sort of pi bond electrons)
-
resonance
exists between C=O and C-N
b. Classification of peptides
1.
dipeptide- 2 amino acid residues.
-named from the N-terminal
residue (add –yl instead of –ine) with
C-terminus residue being last
2. tri, tetra, penta, etc. -peptides. (Same game as dipeptides)
3. Oligopeptides – usually contain 12-20
residues.
4.
Polypeptides-
consists of dozens of residues.
2.
Secondary
Structure- Regular patterns that occur due to interactions between the a-amino (-H) and a-carboxyl groups (-O)
a. a-Helix- coiled primary sequence found in many proteins
1. One
turn of the helix represents 3.6 residues (13 atoms along primary chain)
- Commonly referred as the 3.613 helix
2. Each residue extends 1.5 A
along the helix axis
- amounts to 5.4 A per turn (3.6 residues x 1.5 A/residue)-
- this length defines the pitch of the
helix
3. The diameter of the helix is
~6 A
4. Each peptide carbonyl is hydrogen bonded to the
peptide (N—H) group four
residues farther up the chain.
5. Each side chain is
oriented from the helix axis
6. Each peptide bond has a dipole moment, therefore with the uniform alignment of
carboxyl oxygen, the helix itself has a substantial dipole moment with a positive
charge at the N-terminus and a negative charge at the C-terminus.
7. Other helical structures
a. 310 helix
b. 27 ribbon
c. 4.416 helix (p helix)
b. b-sheet
1. side-by-side peptide strands with H-bonds that form a “pleated sheet”
- the a-Carbons are found along the pleats of the sheet.
2. Peptides can align in two patterns
a. Parallel orientation- where peptides run in the same direction
(1st peptide strand is CàN and adjacent peptide strand is CàN)
-these are more common
-usually larger structures- consisting many times of more than 5 strands
-distributes hydrophobic side chains on both sides of sheet
b. Anti-Parallel orientation- where peptides run in opposite directions
(1st peptide strand is CàN and adjacent peptide strand is NàC)
-these are less common
-typically smaller structures- as few as 2 strands
-distributes hydrophobic side chains on only one side of the sheet.
c. b-Turn or b-bends
peptide chain forms a sharp bend due to hydrogen bonding of the carbonyl of one residue to the amide of a residue three positions down the chain.
-typically proline or glycine are found in the b-bend, due to the conformations of the side chain structures.
3. Tertiary Structuring
-Defines the conformation (overall shape) the polypeptide takes.
The conformation that a protein/polypeptide takes is due simply to stabilization. Due to:
1. formation of large number of intra-molecular hydrogen bonds between secondary
structures
2. reduction in surface area accessible to a solvent (hydrophobic regions)
a. Disulfide bridges- produced by Cysteine residues
b. Hydrogen bonding- interaction between due to polar or charged side chains
c. Hydrophobic interactions- due to hydrophobic side chains
d. Salt bridges- due to charged side chains
4. Quaternary Structures
-Three dimensional structure created by interactions of individual peptide sequences (subunits).
a. Types of quaternary structures
1. Dimer- protein consisting 2 polypeptides sequences
2. Trimer, Tetramer, etc.- protein consisting of more than 2 polypeptide sequence
b. Composition
1. homomers- each subunit is identical
2. heteromers- the subunits are different
E. Protein Architecture
There exist 3 major shapes of proteins based on the general conformation and solubility
I. Fibrous proteins
a. characteristics
1. polypeptide chains are organized parallel along a single axis
2. contain long fibers or large sheets
3. mechanically strong
4. resistant to solubilization in water and dilute salt solutions.
b. examples
1. a-Keratin
a. dominated by a-helices
b. consist of coiled coils-
- a-helix
- coiled coil (2 a-helices)
- protofilament (2 coiled coils)
- filament (4 protofilaments)
c. each a-helix consists of quasi-repeating seven-residue segment (a-b-c-d-e-f-g)
- residues a & d consist of nonpolar residues. These will be oriented to the interior of the helix and therefore stabilizes the a-helix.
d. some forms of a-helices take advantage of disulfide bridges between helices.
Like that hair (curls)
e. found in claws, fingernails, hair and horns
2. Fibroin- (b-sheets in silk)
- composed of tightly bound b-sheets where each peptide strand contains alternating glycine residues. This allows other chains to come much closer (those that contain alanine or serine).
3. Collagen – A triple helix
a. basic structural unit is called tropocollagen (contains 3 wound peptides)
- don’t form true a-helixes due to the high proline content (30%)
- contains hydroxyprolines (3 & 4)
b. every third residue is Gly- creates the hydrogen bonding between peptides
c. very rigid and inextensible protein
d. found in connective tissue of animals
II. Globular Proteins
-generally are spherical in shape and much more numerous
-consist of large amount of a-helices and b-sheets
-pack secondary structures so that only 25% of available volume is space (cavities)
a. examples- almost all enzymes are globular- creation of active site (cavity)
III. Membranous proteins
-membranes that span cellular membrane. Usually have a-helices that span the membrane.