| Vinylation | Vinyl is a common name used to describe an ethylene (ethene) that is attached to another atom/molecule. One example of this is polyvinylchloride (PVC). | |||
|
|
||||
|
Vinyl functional group. The R-labeled atom on the lower left represents any atom or compound. |
||||
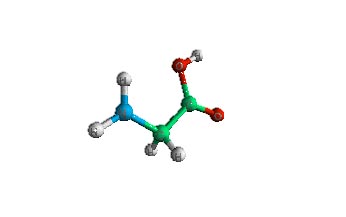
| Amino Acids | Amino Acids are the building blocks of all proteins. There exist 20 common amino acids and many more less common. All amino acids contain 5 parts; central (alpha) carbon, carboxyl (COOH), amino (NH2), a hydrogen, and a side chain (R). | |||
 |
 |
|||
| The amino acid Glycine. Glycine contains a lone hydrogen as the side chain. Note the two Hs pointing down from the central carbon. | The amino acid Alanine. Note the Methyl group (-CH3) pointing down from the central carbon. | |||
| Enantiomerism | Enantiomers are molecules who are stereoisomers that are mirror images of one another. | |||
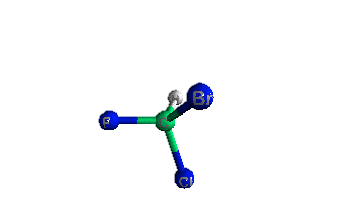
|
|
|
|||
| The R-enantiomeric form of bromochlorofluoromethane | The S-enantiomeric form of bromochlorofluoromethane | |||
| The R refers to a clockwise order of priority from bromine to chlorine to fluorine, whereas the S refers to the counterclockwise order of priority from bromine to chlorine to fluorine. Click here for more. | ||||
| The Berkowitz Lab is interested in specific enantiomeric forms of the vinyl amino acids. Enantiomeric forms have properties that are individual to the specific enantiomer, therefore to obtain a specific vinyl amino acid, the stereochemistry becomes essential. | ||||
| Decarboxylase Enzyme | Decarboxylase enzymes are enzymes that remove a carboxyl group (-COOH) from a substrate. These enzymes usually require the assistance of the the coenzyme Pyridoxal-5-Phosphate (Vitamine B6). The coenzyme aids in weakening the a-carbon-carboxyl carbon bond enabling it to break and stabilize then anion formed by several resonance structures. | |||
|
|
 |
|
||
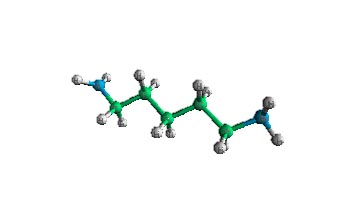
| Here is an example of an a-decarboxylation of lysine ( R= -CH2-CH2-CH2-CH2-NH2). The decarboxylase requires pyridoxal-5-phosphate and a hydrogen atom. The reaction involves removing carbon dioxide and adding a hydrogen. The product of an a-decarboxylation of lysine produces 1,5-diaminopentane. | ||||
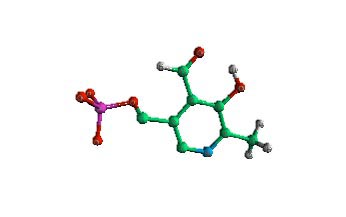 |
The coenzyme pyridoxal-5-phosphate (PLP). This coenzyme forms a stable bond between the amino of an amino acid and the carbonyl on the PLP (top carbon on ring- CHO). | |||